Summary
-
1.
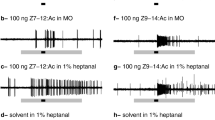
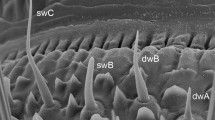
The electrical activity of the two olfactory receptor neurons in individual pheromone-sensitive sensilla on the antennae of male cabbage looper moths (Trichoplusia ni) was monitored extracellularly. Responses to single and multiple component stimuli containing up to three of the seven behaviorally active compounds for this species were obtained at several different stimulus intensities.
-
2.
Neurons which produced large amplitude action potentials (A neurons) were more responsive to (Z)7-dodecenyl acetate, a major component of the female pheromone gland, than were their companion B receptor neurons. B receptor neurons were more responsive to (Z)7-dodecenol, a behavioral inhibitor of male orientation to calling females. Neither neuron was particularly responsive to stimuli containing only dodecyl acetate, a minor component of female glands, which has powerful synergistic effects on male behavior.
-
3.
Some blends of these three compounds elicited responses which were not readily predicted from a knowledge of the cells' responses to individual components of the blend.
-
4.
The average A receptor neuron was significantly more responsive to the blend containing (Z)7-dodecenyl acetate and 10% dodecyl acetate than it was to either component alone or, for that matter, to the algebraic sum of their individual responses. These enhancements were intensity-dependent, occurring to a significant extent only in the middle portion of a neuron's dose-response function. Although A recpetor neurons are not particularly responsive to either (Z)7-dodecenol or dodecyl acetate, a binary mixture of these components elicited significantly smaller responses than expected. Blends which contained all three compounds elicited responses in A receptor neurons which were also significantly smaller than those expected. These reductions were dose-dependent and occurred most reliably at the middle of the dose-response function.
-
5.
The responses of B receptor neurons to blends were more variable than those obtained simultaneously in A receptor neurons. Although all of the various alterations in discharge magnitude observed in the typical A receptor neuron response to blend stimulation were seen in some fraction of the B receptor neurons sampled, only the trinary blend elicited responses which were significantly different from those expected. These reductions in the response of B receptor neurons were also intensity-dependent because they were more reliably observed in the middle portion of the neurons' dose-response function.
-
6.
The sensory processing of complex chemical signals by the insect olfactory system has been postulated to involve a set of narrowly tuned, highly specific olfactory receptor neurons, one for each of the behaviorally relevant component compounds in the pheromone blend. Here we show that olfactory receptor neurons may also be responsive in unique ways to multiple component stimuli even in cases where an individual behaviorally relevant pheromone component is not processed by a separate class of receptor neuron.
Similar content being viewed by others
Abbreviations
- CL :
-
cabbage looper
- DEET :
-
N,N-diethyl-m-to-luamide
- HS :
-
high spontaneous activity
- Z7-12:AC :
-
(Z)7-dodecenyl acetate
- Z7-12: OH :
-
(Z)7-dodecenol
- 12:AC :
-
dodecyl acetate
References
Arn H, Baltensweiler W, Bues R, Buser HR, Esbjerg P, Guerin P, Mani E, Rauscher S, Szocs G, Toth M (1981) Refining lepidopteran sex attractants. In: Les Médiateurs Chimiques. INRA, Versailles, pp 261–265
Baker TC, Carde RT (1979) Courtship behavior of the oriental fruit moth (Grapholitha molesta): Experimental analysis and consideration of the role of sexual selection in the evolution of courtship pheromones in the Lepidoptera. Ann Entomol Soc Am 72:173–188
Baker TC, Carde RT, Roelofs WL (1976) Behavioral responses of maleArgyrotaenia velutinana (Lepidoptera: Tortricidae) to components of its sex pheromone. J Chem Ecol 2:333–352
Baker TC, Gaston LK, Mistrot-Pope M, Kuenen LPS, Vetter RS (1981) A high-efficiency collection device for quantifying sex pheromone volatilized from female glands and synthetic sources. J Chem Ecol 7:961–968
Bartell RJ, Roelofs WL (1973) Inhibition of sexual response in males of the mothArgyrotaenia velutinana by brief exposures to synthetic pheromone or its geometrical isomer. Insect Physiol 19:655–661
Berger RS (1966) Isolation, identification, and synthesis of the sex attractant of the cabbage looper,Trichoplusia ni. Ann Entomol Soc Am 59:767–771
Bjostad LB, Gaston LK, Noble LL, Moyer JH, Shorey HH (1980) Dodecyl acetate, a second pheromone component of the cabbage looper moth,Trichoplusia ni. J Chem Ecol 6:727–734
Bjostad LB, Lin CE, Du JW, Roelofs WL (1984) Identification of new sex pheromone components inTrichoplusia ni, predicted from biosynthetic precursors. J Chem Ecol 10:1309–1323
Bjostad LB, Roelofs WL (1983) Sex pheromone biosynthesis inTrichoplusia ni: key steps involve delta-11 desaturation and chain shortening. Science 220:1387–1389
Bradshaw JWS, Baker R, Lisk JC (1983) Separate orientation and releaser components in a sex pheromone. Nature 304:265–267
Carde RT, Baker TC (1984) Sexual communication with pheromones. In: Bell WJ, Carde RT (eds) Chemical ecology of insects. Chapman and Hall, London, pp 355–383
Davis EE, Sokolove PG (1976) Lactic acid-sensitive receptors on the antennae of the mosquito,Aedes aegypti. J Comp Physiol 105:43–54
Den Otter CJ (1977) Single sensillum responses in the male mothAdoxophyes orana (FvR) to female sex pheromone components and their geometrical isomers. J Comp Physiol 121:205–222
Den Otter CJ, Schuil HA, Sander-Van Oosten A (1978) Reception of host-plant odours and female sex pheromone inAdoxophyes orana (Lepidoptera: Tortricidae): electrophysiology and morphology. Entomol Exp Appl 24:370–378
Dethier VG (1971) A surfeit of stimuli: a paucity of receptors. Am Sci 59:706–715
Fletcher-Howell G, Ferro DN, Butkewich S (1983) Pheromone and blacklight trap monitoring of adult European corn borer (Lepidoptera: Pyralidae) in Western Massachusetts. Environ Entomol 12:531–534
Gillary HL (1966) Stimulation of the salt receptor of the blowfly III. The alkali halides. J Gen Physiol 50:359–368
Guerin PM, Arn H, Buser HR, Charmillot PJ (1981) The sex pheromone ofAdoxophyes orana: preliminary findings from a reinvestigation. In: Les Médiateurs Chimiques. INRA, Versailles, pp 276–269
Guerin PM, Arn H, Blaser C, Lettere M (1983) Z,E-8,10-dode-cadien-1-ol, attractant for malePammene rhediella. Entomol Exp Appl 33:346–347
Ignoffo CM, Berger RS, Graham HM, Martin DF (1963) Sex attractant of cabbage looper,Trichoplusia ni (Hubner). Science 141:902–903
Kaissling KE, Thorson J (1980) Insect olfactory sensilla: structural, chemical and electrical aspects of the functional organization. In: Satelle DB et al. (eds) Receptors for neurotransmitters, hormones and pheromones in insects. Elsevier/North-Holland Biomedical Press, New York, pp 261–282
Kaissling KE, Klein U, Kramer JJ de, Keil TA, Kanaujia S, Hemberger J (1984) Insect olfactory cells: electrophysiological and biochemical studies. In: Changeux JP et al. (eds) Molecular basis of nerve activity. de Gruyter & Co., Berlin, pp 1–11
Klun JA, Chapman OL, Mattes KC, Wojtkowski PW, Beroza M, Sonnet PE (1973) Insect sex pheromones: minor amount of opposite geometrical isomer critical to attraction. Science 181:661–663
Klun JA, Plimmer JR, Bierl-Leonhardt BA, Sparks AN, Chapnman OL (1979) Trace chemicals: the essence of sexual communication systems inHeliothis species. Science 204:1328–1330
Kuenen LPS, Baker TC (1981) Habituation versus sensory adaptation as the cause of reduced attraction following pulsed and constant sex pheromone pre-exposure inTrichoplusiani. J Insect Physiol 27:721–716
Leppla NC (1983) Chemically motivated reproductive isolation between cabbage looper and soybean looper moths (Lepidoptera: Noctuidae). Environ Entomol 12:1760–1765
Light DM, Birch MC (1979) Electrophysiological basis for the behavioral response of male and femaleTrichoplusia ni to synthetic female pheromone. J Insect Physiol 25:161–167
Linn CE, Bjostad LB, Du JW, Roelofs WL (1984) Redundancy in a chemical signal: behavioral responses of maleTrichoplusia ni to a 6-component sex pheromone blend. J Chem Ecol 10:1635–1658
Mayer MS (1973) Electrophysiological correlates of attraction inTrichoplusia ni. J Insect Physiol 19:1191–1198
Mayer MS, Mankin RW, Carlyse TC (1981) External antennal morphometry ofTrichoplusia ni (Hubner) (Lepidoptera: Noctuidae). J Insect Morphol Embryol 10:185–201
McLaughlin JR, Mitchell ER, Chambers DL, Tumlinson JH (1974) Perception of Z-7-dodecen-1-ol and modification of the sex pheromone response of male loopers. Environ Entomol 3:677–680
Meijer GM, Ritter FJ, Persoons CJ, Minks AK, Voerman S (1972) Sex pheromones of summer fruit Tortrix mothAdoxophyes orana: two synergistic isomers. Science 175:1469–1470
Minks AK, Roelofs WL, Schuurmans-Van Dijk E, Persoons CJ, Ritter FJ (1974) Electroantennogram responses of two tortricid moths using two-component sex pheromones. J Insect Physiol 20:1659–1665
O'Connell RJ (1972) Responses of olfactory receptors to the sex attractant, its synergist and inhibitor in the red-banded leaf roller,Argyrotaenia velutiana. In: Schneider D (ed) Olfaction and taste IV. Wissenschaftliche Verlagsgesellschaft, Stuttgart, pp 180–186
O'Connell RJ (1975) Olfactory receptor responses to sex pheromone components in the redbanded leafroller moth. J Gen Physiol 65:179–205
O'Connell RJ (1977) From insect to mammal: complications of the bioassay. In: Müller-Schwarze D, Mozell MM (eds) Chemical signals in vertebrates. Plenum Publishing Corp, New York, pp 377–389
O'Connell RJ (1981) The encoding of behaviorally important odorants by insect chemosensory receptor neurons. In: Norris DM (ed) Perception of behavioral chemicals. Elsevier/ North-Holland Biomedical Press, New York, pp 133–163
O'Connell RJ (1985a) Chemical communication in invertebrates. Experientia (in press)
O'Connell RJ (1985b) Electrophysiological responses to pheromone blends in single olfactory receptor neurons. In: Payne TL, Birch MC, Kennedy CEJ (eds) Mechanisms of perception and orientation to insect olfactory signals. Oxford University Press, Oxford (in press)
O'Connell RJ, Mozell MM (1969) Quantitative stimulation of frog olfactory receptors. J Neurophysiol 32:51–63
O'Connell RJ, Kocsis WA, Schoenfeld RL (1973) Minicomputer identification and timing of nerve impulses mixed in a single recording channel. Proc IEEE 61:1615–1621
O'Connell RJ, Grant AJ, Mayer MS, Mankin RW (1983) Morphological correlates of differences in pheromone sensitivity in insect sensilla. Science 220:1408–1410
Parsons DL, Vallner JJ (1978) Theoretical models for cooperative binding I, II, III. Math Biosci 41:189–240
Priesner E (1979a) Progress in the analysis of pheromone receptor systems. Ann Zool Ecol Anim 11:533–546
Priesner E (1979b) Specificity studies on pheromone receptors of noctuid and tortricid Lepidoptera. In: Ritter FJ (ed) Chemical ecology: odour communication in animals. Elsevier/North-Holland Biomedical Press, New York, pp 57–71
Priesner E (1980) Sensory encoding of pheromone signals and related stimuli in male moths. In: Insect neurobiology and insecticide action (Neurotox 79) Soc Chem Ind, London, pp 359–366
Priesner E (1983) Receptors for di-unsaturated pheromone analogues in the male summerfruit Tortrix moth. Z Naturforsch 38:874–877
Raulston JR, Lopez PP, Gonzales GE, Houghtaling JE (1983) Tobacco budworm: behavioral effects of dispensing virelure or seven-component pheromone in small cotton plots. Environ Entomol 12:738–743
Ritter FJ (1979) Chemical ecology: odour communication in animals. General introduction and overview. In: Ritter FJ (ed) Chemical ecology: odour communication in animals. Elsevier/North-Holland Biomedical Press, New York, pp 1–5
Sass H (1983) Production, release and effectiveness of two female sex pheromone components ofPeriplaneta americana. J Comp Physiol 152:309–317
Siegel S (1956) Nonparametric statistics for the behavioral sciences. McGraw-Hill, New York
Tamaki Y (1979) Multi-component sex pheromone of Lepidoptera with specieal reference toAdoxophyes sp. In: Ritter FJ (ed) Chemical ecology: odour communication in animals. Elsevier/North-Holland Biomedical Press, New York, pp 169–180
Toba HH, Green N, Kishaba AN, Jacobson M, Debolt JW (1970) Response of male cabbage loopers to 15 isomers and congeners of the looper pheromone. J Econ Entomol 63:1048–1051
Tumlinson JH, Teal PEA (1982) The sophisticated language of insect chemical communication. J Georgia Entomol Soc 17:11–23
Tumlinson JH, Mitchell ER, Browner SM, Mayer MS, Green N, Hines R, Lindquist DA (1972) Cis-7-dodecen-1-ol, a potent inhibitor of the cabbage looper sex pheromone. Environ Entomol 1:354–358
Wieczorek H, Köppl R (1982) Reaction spectra of sugar receptors in different taste hairs of the fly. J Comp Physiol 149:207–213
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
O'Connell, R.J. Responses to pheromone blends in insect olfactory receptor neurons. J. Comp. Physiol. 156, 747–761 (1985). https://doi.org/10.1007/BF00610828
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00610828