Summary
-
1.
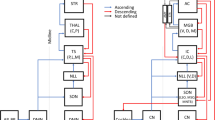
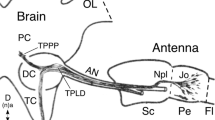
Five interneurones that receive auditory input are described in the Suboesophageal ganglion [SOG] of the locustLocusta migratoria [Fig. 1]. The neurones have been characterised physiologically and anatomically by intracellular recording and staining with Lucifer Yellow.
-
2.
The newly described neurones all have their somata in the SOG (Fig. 1). They are classified into three groups: (a) ascending neurones with axons in the circumoesophageal connectives (SA1, 2, 3); (b) a local neurone with no axon (SL1); and (c) a descending neurone with its axon in the cervical connective (SD1).
-
3.
Transverse sections of the labial and maxillary neuromeres show that the arborisations of the SOG auditory neurones occupy two main areas, medio-dorsal and medio-ventral (Figs. 2, 3). Two auditory interneurones that ascend from the thorax to the brain, the G and B neurones, have segmentally arranged axon collaterals in the SOG (Fig. 1) which project into the same areas of neuropil.
-
4.
In the brain, SA1 terminates in the same dorsal areas of the lower lateral lobe of the protocerebrum as the thoracic ascending auditory interneurones (Fig. 4). SA2 and SA3 terminate more medially where local and descending interneurones arborise.
-
5.
The SOG neurones show sigmoid intensity-response curves (Fig. 7) and broad-band frequency-response curves with maxima between 10 and 20 kHz (Fig. 6), similar to those of the thoracic ascenders at comparable intensities. The SOG neurones have spiking thresholds for auditory stimuli somewhat higher than those of thoracic ascending neurones.
-
6.
All neurones except SD1 spike in response to tones. Discharges are phasic or phasic/tonic in response to short (20 ms) tones, but can become tonic when stimulated with longer tones and depending on the extent of habituation. The SD1 spiked in response to sound signals only with concomitant depolarisation by current injection (Fig. 8B). The oscillating nature of the responses to tones in SD1 (Fig. 8 Biv) and SA2 (Figs. 10B, 11) suggest a recurrent pathway, perhaps involving axon collaterals.
-
7.
Auditory information is conducted from the thorax to the SOG as spikes, not as passively conducted potentials (Fig. 5). Spike latencies in the SOG neurones are long (37–85 ms) compared with those of thoracic ascenders recorded in the SOG (Table 1). In SL1, however, the delay between the spike in a thoracic ascender and the first stimulusrelated EPSP is much shorter (ca. 1 ms) making it a likely candidate to receive information directly from through-fibres.
-
8.
In SA2, SA3, SL1 and SD1, light stimuli also produce EPSPs (Fig. 10) which are not influenced by EPSPs to sound because responses to light and to sound decrement independently. The visual input to ascending SOG neurones indicates there are information loops between the brain and SOG.
-
9.
The responses of SA2, SA3, SL1 and SD1, but not SA1, decrement to varying degrees on repetitive stimulation. The SA3 neurone, which has axons in both circumoesophageal connectives (Fig. 1), displays side-dependent habituation.
-
10.
The neurones described here are components of a previously unknown auditory processing centre in the locust CNS. Their anatomical and physiological properties suggest that the role of the SOG in the auditory pathway needs reassessing, and the concept of the locust auditory system as a linear hierarchy may no longer be valid.
Similar content being viewed by others
Abbreviations
- [a]:
-
Anatomical
- CC :
-
cervical connective
- COC :
-
circumoesophageal connective
- CNS :
-
central nervous system
- LbN :
-
labial nerve
- MdN :
-
mandibular nerve
- MxN :
-
maxillary nerve
- SOG :
-
suboesophageal ganglion
- SupraOG :
-
supraoesophageal ganglion
- SA1–3 :
-
suboesophageal ascending neurones 1–3
- SL1 :
-
suboesophageal local neurone 1
- SD1 :
-
suboesophageal descending neurone 1; Segments of SOG:
- Lb :
-
Labial
- Md :
-
Mandibular
- mx :
-
Maxillary. Tracts in SOG:
- DIT :
-
dorsal intermediatetract
- DMT :
-
dorsal median tract
- LDT :
-
lateral dorsal tract
- MDT :
-
median dorsal tract
- MVT :
-
median ventral tract
- VIT :
-
ventral intermediate tract
- VLT :
-
ventral lateral tract
- VMT :
-
ventral median tract. Commissures in SOG:
- DC I–VI :
-
dorsal commissures I–VI
- VNC :
-
ventral nerve cord. [b] Physiological
- EPSP :
-
excitatory postsynaptic potential
- IPSP :
-
inhibitory postsynaptic potential
- SPL :
-
sound pressure level
References
Abrams TW, Pearson KG (1982) Effects of temperature on identified central neurons that control jumping in the grasshopper. J Neurosci 2:1538–1553
Altman JS, Kien J (1979) Suboesophageal neurons involved in head movements and feeding in locusts. Proc R Soc Lond B 205:209–227
Altman JS, Kien J (1984) The anatomical basis for intersegmental and bilateral coordination in insects. In: Bush B, Clarac F (eds) Coordination of motor behaviour. Soc Exp Biol Seminar Ser 24. Cambridge University Press, Cambridge London (in press)
Altman JS, Shaw MK, Tyrer NM (1980) Input synapses on to a locust sensory neurone revealed by cobalt-electron microscopy. Brain Res 189:245–250
Bentley DR (1969) Intracellular activity in cricket neurones during the generation of behaviour patterns. J Insect Physiol 15:677–699
Boyan GS (1983) Postembryonic development in the auditory system of the locust. Anatomical and physiological characterisation of interneurones ascending to the brain. J Comp Physiol 151:499–513
Boyan GS (1984) Neural mechanisms of auditory information processing by identified interneurones in Orthoptera. J Insect Physiol 30:27–41
Boyan GS (1985) Response decrement in an auditory neurone of the locust. J Insect Physiol (in press)
Brady J (1967) Histological observations on circadian changes in the neurosecretory cells of cockroach sub-oesophageal ganglia. J Insect Physiol 13:201–213
Camhi JM, Hinkle M (1972) Attentiveness to sensory stimuli: central control in locusts. Science 175:550–553
Delcomyn F, Daley DL (1979) Central excitation of cockroach giant interneurones during walking. J Comp Physiol 130:39–48
Eichendorf A, Kalmring K (1980) Projections of auditory ventral-cord neurons in the supraoesophageal ganglion ofLocusta migratoria. Zoomorphologie 94:133–149
Honegger HW, Altman JS, Kien J, Muller-Tautz R, Pollerberg E (1984) A comparative study of neck motor neurones in a cricket and a locust. J Comp Neurol 230:517–535
Horn G (1967) Neuronal mechanisms of habituation. Nature 215:707–711
Kalmring K (1971) Akustische Neuronen im Unterschlundganglion der WanderheuschreckeLocusta migratoria. Z Vergl Physiol 72:95–110
Kalmring K (1975) The afferent auditory pathway in the ventral cord ofLocusta migratoria (Acrididae). I. Synaptic connectivity and information processing among the auditory neurons of the ventral cord. J Comp Physiol 104:103–141
Kalmring K, Rheinlaender J, Rehbein H (1972a) Akustische Neuronen im Bauchmark der WanderheuschreckeLocusta migratoria. Z Vergl Physiol 76:314–332
Kalmring K, Rheinlaender J, Römer H (1972b) Akustische Neuronen im Bauchmark vonLocusta migratoria. Der Einfluß der Schallrichtung auf die Antwortmuster. J Comp Physiol 80:325–352
Kalmring K, Kühne R, Moysich F (1978a) The auditory pathway in the ventral cord of the migratory locust (Locusta migratoria): response transmission in the axons. J Comp Physiol 126:25–33
Kalmring K, Kühne R, Moysich F (1978b) The coding of sound signals in the ventral-cord auditory system of the migratory locust,Locusta migratoria. J Comp Physiol 128:213–226
Kien J (1983) The initiation and maintenance of walking in the locust: an alternative to the command concept. Proc R Soc Lond B 219:137–174
Kien J, Altman JS (1984) Descending interneurons from the brain and suboesophageal ganglia and their role in the control of locust behaviour. J Insect Physiol 30:59–72
Kien J, Williams M (1983) Morphology of neurons in locust brain and suboesophageal ganglion involved in initiation and maintenance of walking. Proc R Soc Lond B 219:175–192
O'Shea M (1975) Two sites of axonal spike initiation in a bimodal interneuron. Brain Res 96:93–98
O'Shea M, Rowell CHF, Williams JLD (1974) The anatomy of a locust visual interneurone; the descending contralateral movement detector. J Exp Biol 60:1–12
Otto D, Campan R (1978) Descending interneurons from the cricket suboesophageal ganglion. Impulse patterns correlated with ventilation. Naturwissenschaften 65:491–492
Otto D, Weber T (1982) Interneurons descending from the cricket cephalic ganglia that discharge in the pattern of two motor rhythms. J Comp Physiol 148:209–219
Pearson KG, Goodman CS (1979) Correlation of variability in structure with variability in synaptic connections of an identified interneuron in locusts. J Comp Neurol 184:141–166
Pearson KG, Robertson RM (1981) Interneurons coactivating hindleg flexor and extensor motoneurons in the locust. J Comp Physiol 144:391–400
Pearson KG, Heitler WJ, Steeves JD (1980) Triggering of locust jump by multimodal inhibitory interneurons. J Neurophysiol 43:257–278
Peters BH, Altman JS, Tyrer NM (1985) Synaptic connections between the hindwing stretch receptor and flight motor neurones in a locust revealed by double cobalt labelling for electron microscopy. J Comp Neurol (in press)
Rehbein HG (1973) Experimentell-anatomische Untersuchungen über den Verlauf der Tympanalnervenfasern im Bauchmark von Feldheuschrecken, Laubheuschrecken und Grillen. Verh Dtsch Zool Ges 66:184–189
Rehbein H (1976) Auditory neurons in the ventral nerve cord of the locust: morphological and functional properties. J Comp Physiol 110:233–250
Rehbein H, Kalmring K, Römer H (1974) Structure and function of acoustic neurons in the thoracic ventral nerve cord ofLocusta migratoria (Acrididae). J Comp Physiol 95:263–280
Römer H, Marquart V (1984) Morphology and physiology of auditory interneurons in the metathoracic ganglion of the locust. J Comp Physiol A 155:249–262
Rowell CHF (1964) Central control of an insect segmental reflex. I. Inhibition by different parts of the central nervous system. J Exp Biol 41:559–572
Rowell CHF, Horn G (1968) Dishabituation and arousal in the response of single nerve cells in an insect brain. J Exp Biol 49:171–183
Simmons P (1980) A locust wind and ocellar brain neurone. J Exp Biol 85:281–294
Steeves JD, Pearson KG (1982) Proprioreceptive gating of inhibitory pathways to hindleg flexor motorneurones in the locust. J Comp Physiol 146:507–515
Stewart WW (1978) Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell 14:741–759
Tyrer NM, Gregory GE (1982) A guide to the neuroanatomy of locust suboesophageal and thoracic ganglia. Phil Trans R Soc Lond B 297:91–123
Wiersma CAG (1967) Visual central processing in crustaceans. In: Wiersma CAG (ed) Invertebrate nervous systems. University Chicago Press, Chicago, pp 269–284
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boyan, G.S., Altman, J.S. The suboesophageal ganglion: a ‘missing link’ in the auditory pathway of the locust. J. Comp. Physiol. 156, 413–428 (1985). https://doi.org/10.1007/BF00610734
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00610734