Abstract
We have investigated the role of the electrogenic hydrogen ion pump in the regulation of intracellular sodium ion activity (a iNa ) and intracellular pH (pHi) in frog skin epithelial cells using double-barreled ion sensitive microelectrodes. WhenRana esculenta skin is mounted in an Ussing chamber and bathed in 1 mM Na2SO4 buffered to pH 7.34 with imidazole on the apical side and in normal Ringer on the serosal side, the apical addition of the carbonic anhydrase inhibitor, ethoxzolamide (10−4M) blocks net H+ ion excretion and Na absorption, producing a depolarization of 25–30 mV of the apical membrane, potential (Ψmc). We demonstrate the these changes are accompanied by a fall ina iNa from 6.2±0.5 mmol/l to 3.4±0.6 mmol/l and an increase in pHi from 7.20±0.03 to 7.38±0.08 (n=12 skins). Voltage clamping Ψmc to its control value in the presence of ethoxzolamide restoreda iNa but the pHi remained alkaline. Furthermore, the fall ina iNa produced by ethoxzolamide could be mimicked by voltage clamping Ψmc towards the value of the Nernst potential for Na at the apical membrane. These results indicate that the maintenance of the cellular Na+ transport pool is dependent on a favourable electrical driving force and counter-current generated by an electrogenic H+ pump at the apical membrane.
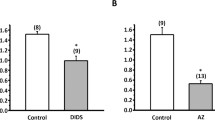
Addition of amiloride (10−5 mol/l) or substitution of external Na+ by Mg2+ or K+ caused a hyperpolarization of Ψmc and a fall ina iNa . These effects were accompanied by an inhibition of H+ excretion and a fall in pHi of 0.14 ±0.08 units (n=6 skins). However, when the effect, of Na+ transport inhibition on Ψmc was prevented by imposing a voltage clamp no effects of amiloride on H+ excretion or pHi were observed. Moreover, the effect of amiloride on pHi could be reproduced in control skins by voltage clamping Ψmc to −100 mV. The metabolic inhibitors vanadate (1 mmol/l) and di-cyclo hexyl carbodiimide (5×10−5 mol/l) inhibited H+ excretion and decreased pHi from 7.28±0.08 to 7.02±0.06 and from 7.30±0.06 to 7.12±0.05 (n=6 skins), respectively.
These results indicate that an apical membrane H+ ATPase plays a role in regulating pHi and the mechanism is sensitive to membrane potential.
Similar content being viewed by others
References
Bowman B, Slayman CW (1977) Characterization of plasma membrane adenosine triphosphatase ofNeurospora crassa. J Biol Chem 252:3357–3363
Bowman B, Mainzer S, Allen K, Slayman CW (1978) Effects of inhibitors in the plasma membrane and mitochondrial adenosine triphosphatases ofNeurospora crassa. Biochem Biophys Acta 512:13–28
Cantley Jr LC, Gosephson L, Warner R, Yanagisawa M, Lechene C, Guidotti G (1977) Vanadate is a potent (Na, K) ATPase inhibitor found in ATP derived from muscle. J Biol Chem 252:7421–7423
Ehrenfeld J, Garcia-Romeu F (1977) Active hydrogen excretion and sodium absorption through isolated frog skin. Am J Physiol 233:F46-F54
Ehrenfeld J, Garcia-Romeu F, Harvey BJ (1985) Electrogenic active proton pump inRana esculenta skin and its role in sodium ion transport. J Physiol (Lond) 359:331–355
Harold FM, Baarda JR (1969) Inhibition of membrane bound adenosine triphosphatase and cation transport ofStaphylococcus faecalis By N,N1-dicyclohexylcarbodiimide. J Biol Chem 244:2261–2268
Harvey BJ, Kernan RP (1984) Intracellular ionic activities in frog skin in relation to external sodium concentration and in the presence of amiloride and/or ouabain. J Physiol (Lond) 349:501–517
Maren TH (1967) Carbonic anhydrase: chemistry, physiology and inhibition. Physiol Rev 47:618–635
Roos A, Boron WF (1981) Intracellular pH. Physiol Rev 61: 296–434
Sachs G, Spenney JG, Lewin M (1978) H+ transport regulation and mechanism in gastric mucosa and membrane vesicles. Physiol Rev 58:106–173
Soltoff SP, Mandel LJ (1983) Amiloride directly inhibits Na, K-ATPase activity of rabbit proximal tubules. Science 220 (4500):959–961
Steinmetz PR, Husted RF, Mueller A, Beauwens R (1981) Coupling between H+ transport and anaerobic glycolysis in turtle urinary bladder: effects of inhibitors of H+ ATPase. J Membr Biol 59:27–34
Steinmetz PR, Andersen OS (1982) Electrogenic proton transport in epithelial membranes. J Membr Biol 6:155–174
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Harvey, B.J., Ehrendeld, J. Regulation of intracellular sodium and pH by the electrogenic H+ pump in frog skin. Pflugers Arch. 406, 362–366 (1986). https://doi.org/10.1007/BF00590937
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00590937