Summary
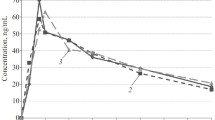
In a double-blind, placebo-controlled study in 6 healthy volunteers, the correlation between beta-adrenoceptor binding, the time course of the effect and plasma concentration kinetics was investigated from 0 to 48 h after a single oral dose of propranolol 240 mg. First, the in vitro beta-adrenoceptor interaction of propranolol was investigated. Propranolol inhibited beta-adrenoceptor binding to rat parotid (beta1) and reticulocyte (beta2) membranes in the presence of pooled human plasma with a Ki of about 8 ng/ml plasma. After oral administration of 240 mg propranolol, concentration kinetics in plasma could be described by a Bateman function with a fictive concentration at time 0 of 275 ng/ml plasma, and a mean elimination half-life of 3.5 h. Using the concentration kinetics of propranolol in plasma together with its in vitro beta-adrenoceptor binding characteristics in the presence of placebo plasma from each individual, the time course of antagonism against beta-adrenoceptor mediated effects was predicted. The latter was in agreement with the time course of propranolol-induced inhibition of tachycardia due to orthostasis. After bicycle ergometry, however, the time course of inhibition of tachycardia was shorter than was predicted. Plasma sampled at various times after propranolol administration inhibited beta-adrenoceptor binding of the radioligand 3H-CGP 12177 to rat reticulocyte membranes in a fashion reflecting the time course of inhibition of exercise tachycardia observed in the volunteers. A direct, linear relation was shown between the in vitro inhibition of beta-adrenoceptor binding by the plasma samples withdrawn after propranolol administration and the inhibition of exercise tachycardia observed in parallel. The results show that the concentrations of antagonist present in plasma are representative of the concentrations in the effect compartment. Deep compartments of drug distribution appear irrelevant to the effects of the drugs. The relation between the plasma concentration of propranolol and the reduction in heart rate at various levels of physical effort shows no significant inhibition at rest and increasing IC50-values from orthostasis to 2 min and to 4 min of ergometry. IC50-values after orthostasis are in the range of the Ki-values from in vitro receptor binding studies, whereas the IC50-values after exercise are shifted 2-to 3-fold to the right relative to the Ki-values. This finding is in agreement with increased beta-adrenoceptor stimulation with increasing effort (release of endogenous noradrenaline), which shifts the antagonist concentration-effect curve to the right. Furthermore, the rightward shift can explain why with increasing effort the time course of the inhibitory effect of propranolol becomes shorter. Release of propranolol from presynaptic stores during exercise is irrelevant, since this would result in opposed effects on the concentration-effect relationship (leftward shift) and the time course of antagonism (longer effect) with increasing work load. It is concluded that the receptor interaction of propranolol together with its plasma concentration kinetics can fully explain the time course of effects after a single oral dose, and so receptor interaction will be the missing link in the correlation between concentration kinetics and effect kinetics of propranolol in man. In general, this mode of correlation should be expandable to any drug exerting its effects according to the law of mass action via receptors in the extracellular space. This approach provides a rational basis for the comparison of different drugs from one group irrespective of their receptor affinity and concentration kinetics.
Similar content being viewed by others
References
Ariëns EJ (1984) Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology. Eur J Clin Pharmacol 26: 663–668
Arunlakshana O, Schild HO (1959) Some quantitative uses of drug antagonists. Br J Pharmacol 14: 48–58
Bonelli (1979) Beta-Rezeptoren-Blockade. Klinische Pharmakologie und klinisch therapeutische Anwendung. Springer, Wien New York
Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB (1982) Decreased catecholamine sensitivity and β-adrenergic-receptor density in failing human hearts. N Engl J Med 307: 205–211
Broadley KJ (1982) Cardiac adrenoceptors. J Auton Pharmacol 2: 119–145
Brodde OE, Engel G, Hoyer D, Bock KD, Weber F (1981) The β-adrenergic receptor in human lymphocytes: Subclassification by the use of a new radio-ligand, (+/-)125-Iodocyanopindolol. Life Sci 29: 2189–2198
Brodde OE, Karad K, Zerkowski HR, Rohm N, Reidemeister JC (1983) Coexistence of β1- and β2-adrenoceptors in human right atrium. Direct identification by (+/-)125-Iodocyanopindolol binding. Circ Res 53: 752–758
Chamberlain DA, Turner P, Sneddon JM (1967) Effects of atropine on heart-rate in healthy man. Lancet 1: 12–15
Chidsey C, Pine M, Favrot L, Smith S, Leonetti G, Morselli P, Zanchetti A (1976) The use of drug concentration measurements in studies of the therapeutic responses to propranolol. Postgrad Med J 52 [Suppl 4]: 26–32
Colburn WA (1981) Simultaneous pharmacokinetic and pharmacodynamic modeling. J Pharmacokinet Biopharm 9: 367–388
Coltart DJ, Shand DG (1970) Plasma propranolol levels in the quantitative assessment of β-adrenergic blockade in man. Br Med J 3: 731–734
Cuthbert MF, Collins RF (1975) Plasma levels and β-adrenoceptor blockade with acebutolol, practolol and propranolol in man. Br J Clin Pharmacol 2: 49–55
Daniell HB, Walle T, Gaffney TE, Webb JG (1979) Stimulation-induced release of propranolol and norepinephrine from adrenergic neurons. J Pharmacol Exp Ther 208: 354–359
De Lean A, Hancock AA, Lefkowitz RJ (1982) Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. Mol Pharmacol 21: 5–16
Delhaye M, Taton G, Camus JC, Chatelain P, Robberecht P, Waelbroeck M, Christophe J (1983) Effects of full and partial β-adrenergic agonists and antagonists on human lung adenylate cyclase. Biochem Pharmacol 32: 1831–1835
Fitzgerald JD (1980) Propranolol. In: A Scriabine (ed) Pharmacology of antihypertensive drugs. Raven Press, New York, pp 195–208
Fleming WW, Westfall DP, De la Lande IS, Jellett LB (1972) Lognormal distribution of equieffective doses of norepinephrine and acetylcholine in several tissues. J Pharmacol Exp Ther 181: 339–345
Gladtke E, v Hattingberg HM (1977) Pharmakokinetik, (2nd edn). Springer, Berlin Heidelberg New York
Gugler R, Krist K, Raczinski H, Höffgen H, Bodem G (1980) Comparative pharmacodynamics and plasma levels of β-adrenoceptor blocking drugs. Br J Clin Pharmacol 10: 337–343
Hager WD, Pieniascek HJ, Perrier D, Mayersohn M, Goldberger V (1981) Assessment of beta blockade with propranolol. Clin Pharmacol Ther 30: 283–290
Harms HH (1976) Isoproterenol antagonism of cardioselective β-adrenergic receptor blocking agents: A comparative study of human and guinea-pig cardiac and bronchial β-adrenergic receptors. J Pharmacol Exp Ther 199: 329–335
v Hattingberg HM, Brockmeier D, Kreuter G (1977) A rotating iterative procedure (RIP) for estimating hybrid constants. Eur J Clin Pharmacol 11: 381–388
van Herwaarden CLA, Fennis JFM, Binkhorst RA, v'ant Laar A (1977) Haemodynamic effects of adrenaline during treatment of hypertensive patients with propranolol and metoprolol. Eur J Clin Pharmacol 12: 397–402
Hiatt WR, Fradl DC, Zerbe GO, Byyny RL, Nies AS (1984) Selective and nonselective β-blockade of the peripheral circulation. Clin Pharmacol Ther 35: 12–18
Holford NH, Sheiner LB (1982) Kinetics of pharmacologic response. Pharmacol Ther 16: 143–166
Hui KKP, Conolly ME, Tashkin DP (1982) Reversal of human lymphocyte β-adrenoceptor desensitization by glucocorticoids. Clin Pharmacol Ther 32: 566–576
Hurwitz GA, Webb JG, Walle T, Bai SA, Daniell HB, Gourley L, Loadholt CB, Gaffney TE (1983) Exercise-induced increments in plasma levels of propranolol and noradrenaline. Br J Clin Pharmacol 16: 599–608
Ishizaki T, Tawara K (1978) Comparison of disposititon and effect to timolol and propranolol on exercise tachycardia. Eur J Clin Pharmacol 14: 7–14
Ishizaki T (1980) Likely explanation for longer duration of pharmacological (antianginal) effects of propranolol in relation to its short half-life. Res Commun Chem Pathol Pharmacol 27: 223–239
Israili ZH (1979) Correlation of pharmacological effects with plasma levels of antihypertensive drugs in man. Ann Rev Pharmacol Toxicol 19: 25–52
Kelman AW, Whiting B (1980) Modeling of drug response in individual subjects. J Pharmacokinet Biopharm 8: 115–130
Kopin IJ, Zukowska-Grojec Z, Bayorh MA, Goldstein DS (1984) Estimation of intrasynaptic norepinephrine concentrations at vascular neuroeffector junctions in vivo. Naunyn-Schmiedeberg's Arch Pharmacol 325: 298–305
de Leede GJL, Hug CC, de Lange S, de Boer AG, Breimer DD (1984) Rectal and intravenous propranolol infusion to steady state: Kinetics and β-receptor blockade. Clin Pharmacol Ther 35: 148–155
Lemmer B, Bathe K, Lang PH, Neumann G, Winkler H (1983) Chronopharmacology of β-adrenoceptor-blocking drugs: Pharmacokinetic and pharmacodynamic studies in rats. J Am Coll Toxicol 2: 347–357
Levy G (1966) Kinetics of pharmacologic effects. Clin Pharmacol Ther 7: 362–372
Levy G, Gibaldi M (1975) Pharmacokinetics. In: JR Gillette, JR Mitchell, P Randall (eds) Concepts in biochemical pharmacology, part 3. Springer, Berlin Heidelberg New York, pp 4–34
McAinsh J, Baber NS, Smith R, Young J (1978) Pharmacokinetic and pharmacodynamic studies with long-acting propranolol. Br J Clin Pharmacol 6: 115–121
McDevitt DG, Shand DG (1975) Plasma concentrations and the time-course of β-blockade due to propranolol. Clin Pharmacol Ther 18: 708–713
Mullane JF, Kaufman J, Dvornik D, Coelho J (1982) Propranolol dosage, plasma concentration, and β-blockade. Clin Pharmacol Ther 32: 692–700
Okupa FE, Daneshmend TK, Shrosbree E, Roberts CJC (1981) Dose response studies of indenolol, a β-adrenoceptor blocker. Clin Pharmacol Ther 29: 434–439
Platzer R, Galeazzi RL, Niederberger W, Rosenthaler J (1984) Simultaneous modeling of bopindolol kinetics and dynamics. Clin Pharmacol Ther 36: 5–13
Robberecht P, Delhaye M, Taton G, De Neef P, Waelbroeck M, De Smet JM, Leclerc JL, Chatelain P, Christophe J (1983) The human heart β-adrenergic receptors. I. Heterogeneity of the binding sites: Presence of 50% β1- and 50% β2-adrenergic receptors. Mol Pharmacol 24: 169–173
Russell PM, Webb JG, Walle T, Daniell HB, Privitera PJ, Gaffney TE (1983) Adrenergic nerve stimulation-induced release of propranolol from the perfused hindlimb and spleen of the dog and associated changes in postjunctional response. J Pharmacol Exp Ther 226: 324–329
Scriabine A (1979) β-adrenoceptor blocking drugs in hypertension. Ann Rev Pharmacol Toxicol 19: 269–284
Serlin MJ, Orme LE, Baber NS, Sibeon RG, Laws E, Breckenridge A (1980) Propranolol in the control of blood pressure: A dose-response study. Clin Pharmacol Ther 27: 586–592
Stachelin M, Simons P, Jaeggi K, Wigger N (1983) CGP 12177. A hydrophilic β-adrenergic receptor radioligand reveals high affinity binding of agonists to intact cells. J Biol Chem 258: 3496–3502
Stiles GL, Taylor S, Lefkowitz RJ (1983a) Human cardiac β-adrenergic receptors: Subtype heterogeneity delineated by direct radioligand binding. Life Sci 33: 467–473
Stiles GL, Strasser RH, Lavin TN, Jones LR, Caron MC, Lefkowitz RJ (1983b) The cardiac β-adrenergic receptor. Structural similarities of β1 and β2-receptor subtypes demonstrated by photoaffinity labeling. J Biol Chem 258: 8443–8449
Street JA, Webb JG, Bright S, Gaffney TE (1984) Accumulation, subcellular localization and release of propranolol from synaptosomes of rat cerebral cortex. J Pharmacol Exp Ther 229: 154–161
Vukovich A, Foley JE, Brown B, Willard DA, Buckley M, O'Kelly D, Fitzgerald D, Tormey W, Darragh A (1979) Effects of β-blockers on exercise double product (systolic blood pressure × heart rate). Br J Clin Pharmacol 7 [Suppl 2]: 167S-172S
Waelbroeck M, Taton G, Delhaye M, Chatelain P, Camus JC, Pochet R, Leclerc JL, De Smet JM, Robberecht P, Christophe J (1983) The human heart β-adrenergic receptors. II. Coupling of β2-adrenergic receptors with the adenylate cyclase system. Molec Pharmacol 24: 174–182
Wellstein A, Jablonka B, Wiemer G, Palm D (1984a) Increase of Bmax-values of β-adrenoceptor sites after chronic treatment with reserpine? Pol J Pharmacol Pharm 36: 261–269
Wellstein A, Palm D, Wiemer G, Schäfer-Korting M, Mutschler E (1984b) Simple and reliable radioreceptor assay for β-adrenoceptor antagonists and active metabolites in native human plasma. Eur J Clin Pharmacol 27: 545–553
Wellstein A, Palm D, Belz GG, Pitschner HF (1985) Receptor binding characteristics and pharmacokinetic properties as a tool for the prediction of clinical effects of β-blockers. Drug Res 35: 2–6
Whiting B, Kelman AW (1980) The modeling of drug response. Clin Sci 59: 311–315
Wiemer G, Wellstein A, Palm D, v Hattingberg HM, Brockmeier D (1982) Properties of agonist binding at the β-adrenoceptor of the rat reticulocyte. Naunyn-Schmiedeberg's Arch Pharmacol 321: 11–19
Zacest R, Koch-Weser J (1972) Relation of propranolol plasma level to β-blockade during oral therapy. Pharmacology 7: 178–184
Author information
Authors and Affiliations
Additional information
This work is dedicated to Prof. H. J. Schümann on the occasion of his 65th birthday
Rights and permissions
About this article
Cite this article
Wellstein, A., Palm, D., Pitschner, H.F. et al. Receptor binding of propranolol is the missing link between plasma concentration kinetics and the effect-time course in man. Eur J Clin Pharmacol 29, 131–147 (1985). https://doi.org/10.1007/BF00547412
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00547412