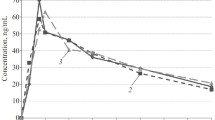
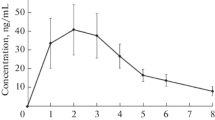
We studied pharmacokinetics and bioavailability of verapamil, propranolol, and ethacizine in healthy volunteers after single oral administration under normal conditions and on the second day of simulated antiorthostatic hypokinesia modeling some effects of microgravity. Under conditions of antiorthostatic hypokinesia, a tendency to a decrease in half-elimination period, mean retention time, and volume of distribution and an increase in the rate of absorption, ratio of maximum concentrations, and relative rate of absorption of verapamil and propranolol were revealed. For ethacizine, a statistically significant increase in the time of attaining maximum concentration and volume of distribution and a decrease in the maximum concentration, rate of absorption, ratio of maximum concentrations, and relative rate of absorption under conditions of antiorthostatic hypokinesia were found.
Similar content being viewed by others
References
Abyshev AZ, Agaev EM, Semenov EV. New-Generation Calcium Ion Antagonists. Baku, 2003. Russian.
Arzamastsev AP, Kondratenko SN, Starodubtsev AK, Blinkov IL, Svetyi LI. Peculiarities of the pharmacokinetics of some cardiovascular drugs in patients with inflammatory disturbances of the large intestine. Pharm. Chem. J. 2003;37(2):60-62.
Beloborodov VL, Zalesskaya MA, Tyukavkina NA, Bugrii EM, Kaverina NN. Clinical pharmacokinetics of ethmozine and ethacizine in the course of combined administration. Pharm. Chem. J. 2004;38(2):59-62.
Goncharov IB, Kovalevich IV. The System of Medical Treatment for Cosmonauts. Orbital Station Mir. Vol. 1. Medical Care in Long Term Space Flight. Moscow, 2001. P. 432-454. Russian.
Goncharov IB, Kovachevich IV, Repenkova LG, Kondratenko SN, Starodubtsev AK. Effect of antiorthostatic hypokinesia on acetaminophen pharmacokinetics and its distribution in saliva of healthy volunteers. Pharm. Chem. J. 2009;43(5):235-238. doi:https://doi.org/10.1007/s11094-009-0288-x
Kovachevich IV, Repenkova LG, Kondratenko SN, Starodubtsev AK. Pharmacokinetics of acetaminophen administered in tablets and capsules under long-term space flight conditions. Pharm. Chem. J. 2009;43(3):130-133.
Kukes VG, Grachev SV, Suchev DA, Ramenskaya GV. The Metabolism of Drugs. Scientific Bases of Personalized Medicine. Moscow, 2008. P. 269. Russian.
Miroshnichenko II, Tyulaev II, Zyev AP. Biocompatibility of Drugs. Moscow, 2003. Russian.
Assessment of the Bioequivalence of Medicinal Products: Guidelines. Moscow, 2008. P. 18-21.
Ramenskaya GV, Kondratenko SN, Krasnykh LM. Quantitative assay of the drugs in the blood plasma of patients by high-performance liquid chromatography. Clinical Pharmacokinetics: Theoretical, Applied, and Analytical Aspects. Kukes VG, ed. Moscow, 2009. P. 396-397. Russian.
Sergienko VI, Jelliffe RW, Bondareva IB. Applied Pharmacokinetics: Basic Foundations and Clinical Implementations. Moscow, 2003. Russian.
Gandia P, Saivin S, Le-Traon AP, Guell A, Houin G. Influence of simulated weightlessness on the intramuscular and oral pharmacokinetics of promethazine in 12 human volunteers. J. Clin. Pharmacol. 2006;46(9):1008-1016.
Idkaidek N, Arafat T. Effect of microgravity on the pharmacokinetics of ibuprofen in humans. J. Clin. Pharmacol. 2011;51(12):1685-1689.
Kast J, Yu Y, Seubert CN, Wotring VE, Derendorf H. Drugs in space: Pharmacokinetics and pharmacodynamics in astronauts. Eur. J. Pharm. Sci. 2017;109S:S2-S8.
Lutz RJ, Dedrick RL, Matthews HB, Eling TE, Anderson MW. A preliminary pharmacokinetic model for several chlorinated bipherel in the rat. Drug Metab. Dispos.1977;5(4):386-396.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 168, No. 10, pp. 449-453, October, 2019
Rights and permissions
About this article
Cite this article
Polyakov, A.V., Svistunov, A.A., Kondratenko, S.N. et al. Peculiarities of Pharmacokinetics and Bioavailability of Some Cardiovascular Drugs under Conditions of Antiorthostatic Hypokinesia. Bull Exp Biol Med 168, 465–469 (2020). https://doi.org/10.1007/s10517-020-04732-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-020-04732-w