Summary
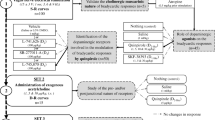
The effect of N-ethylmaleimide (NEM), which has been shown to abolish rather selectively inhibition of adenylate cyclase, on the α2-adrenoceptor modulation of noradrenaline release was studied. Slices of the rabbit hippocampus were loaded with 3H-noradrenaline, superfused continuously and stimulated twice electrically.
NEM (30 μmol/l) applied for 30 min enhanced both basal and stimulation-evoked tritium overflow significantly. Occupation of the receptor by the α2-adrenoceptor agonist clonidine prior to and during NEM treatment did not protect the α2-adrenoceptor-mediated autoinhibitory feedback system from being affected by NEM. Preincubation of the hippocampal slices with NEM was without any influence on 3H-noradrenaline uptake. The inhibitory effect of clonidine on 3H-noradrenaline release was attenuated in a non-competitive manner. In addition, the facilitatory effect of the α2-adrenoceptor antagonist yohimbine on the stimulusevoked tritium overflow was reduced. The facilitation of the evoked noradrenaline release by yohimbine or yohimbine and NEM converged with increasing concentrations of yohimbine, suggesting that yohimbine and NEM were acting at the same signal-transduction system.
These results are compatible with the idea that NEM, by alkylating the Ni-unit of a presynaptically located adenylate cyclase, prevents the α2-adrenoceptor-mediated modulation of noradrenaline release.
Similar content being viewed by others
Abbreviations
- NEM:
-
N-ethylmaleimide
- IAP:
-
islet-activating protein
References
Aktories K, Schultz G, Jakobs KH (1980) Regulation of adenylate cyclase activity in hamster adipocytes. Naunyn-Schmiedeberg's Arch Pharmacol 312:167–173
Allgaier C, Feuerstein TJ, Jackisch R, Hertting G (1985) Islet-activating protein (pertussis toxin) diminishes α2-adrenoceptor-mediated effects on noradrenaline release. Naunyn-Schmiedeberg's Arch Pharmacol 331:235–239
Cooper DF (1982) Bimodal regulation of adenylate cyclase. FEBS Lett 138:157–163
Cubeddu LX, Barnes E, Weiner N (1975) Release of norepinephrine and dopamine-β-hydroxylase by nerve stimulation. IV. An evaluation of a role for cyclic adenosine monophosphate. J Pharmacol Exp Ther 193:105–127
Dolphin AC, Prestwich SA (1985) Pertussis toxin reverses adenosine inhibition of neuronal glutamate release. Nature 216:148–150
Gilman AG (1984) G proteins and dual control of adenylate cyclase Cell 36:577–579
Harden TK, Scheer AG, Smith MM (1982) Differential modification of the interaction of cardiac muscarinic cholinergic and β-adrenergic receptors with a guanine-nucleotide binding component(s). Mol Pharmacol 21:570–580
Hildebrandt JD, Sekura RD, Codina J, Iyengar R, Manclark CR, Birnbaumer L (1983) Stimulation and inhibition of adenylyl cyclases mediated by distinct regulatory proteins. Nature 302:706–709
Illes P, Rubini P (1984) Mechanisms of inhibition by opioids and α2-adrenoceptor agonists of noradrenaline release. In: Vizi ES, Magyar K (eds) Regulation of transmitter function: Basic and clinical aspects. Akademiai Kiado, Budapest, pp 163–173
Jackisch R, Werle E, Hertting G (1984) Identification of mechanisms involved in the modulation of release of noradrenaline in the hippocampus of the rabbit in vitro. Neuropharmacology 23:1363–1371
Jakobs KH, Lasch P, Minuth M, Aktories K, Schultz G (1982) Uncoupling of α-adrenoceptor-mediated inhibition of human platelet adenylate cyclase by N-ethylmaleimide. J Biol Chem 257:2829–2833
Jakobs KH, Aktories K, Schultz G (1984) Mechanisms and components involved in adenylate cyclase inhibition by hormones. Adv Cyclic Nucleotide Res 17:135–143
Kahn DJ, Mitrius JC, U'Prichard DC (1982) Alpha2-adrenergic receptors in neuroblastoma x glioma hybrid cells. Mol Pharmacol 21:17–26
Kawai M, Nomura Y (1983) Involvement of sulfhydryl groups in cerebral cortical 3H-clonidine binding in developing rats. Eur J Pharmacol 91:449–454
Lai RT, Watanabe Y, Yoshida H (1983) Effect of islet-activating protein (IAP) on contractile responses of rat vas deferens: evidence for participation of Ni (inhibitory GTP binding regulating protein) in the α2-adrenoceptor-mediated response. Eur J Pharmacol 90:453–456
Lasch P, Jakobs KH (1979) Agonistic and antagonistic effects of various α-adrenergic agonists in human platelets. Naunyn-Schmiedeberg's Arch Pharmacol 306:119–125
Markstein R, Digges K, Marshall NR, Starke K (1984) Forskolin and the release of noradrenaline in cerebrocortical slices. Naunyn-Schmiedeberg's Arch Pharmacol 325:17–24
Mulder AH, Frankhuyzen AL, Stoof JC, Wemer J, Schoffelmeer ANM (1984) Catecholamine receptors, opiate receptors, and presynaptic modulation of neurotransmitter release in the brain. In: Usdin E, Carlsson A, Dahlström A, Engel J (eds) Catecholamines: Neuropharmacology and central nervous system — theoretical aspects. A. R. Liss, New York, pp 47–58
Murayama T, Ui M (1983) Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem 258:3319–3326
Pelayo F, Dubocovich ML, Langer SZ (1978) Possible role of cyclic nucleotides in regulation of noradrenaline release from rat pineal through presynaptic adrenoceptors. Nature 274:76–78
Schoffelmeer ANM, Mulder AH (1983) 3H-Noradrenaline release from rat neocortical slices in the absence of extracellular Ca2+ and its presynaptic alpha2-adrenergic modulation. Naunyn-Schmiedeberg's Arch Pharmacol 323:188–192
Stjärne L, Bartfai T, Alberts P (1979) The influence of 8-Br 3′,5′-cyclic nucleotide analogs and of inhibitors of 3′,5′-cyclic nucleotide phosphodiesterase, on noradrenaline secretion and neuromuscular transmission in guinea-pig vas deferens. Naunyn-Schmiedeberg's Arch Pharmacol 308:99–105
Ukena D, Poeschla E, Hüttemann E, Schwabe U (1984) Effects of N-ethylmaleimide on adenosine receptors of rat fat cells and human platelets. Naunyn-Schmiedeberg's Arch Pharmacol 327:247–253
Wemer J, Schoffelmeer ANM, Mulder AH (1982) Effects of cyclic AMP analogues and phosphodiesterase inhibitors on K+-induced (3H)-noradrenaline release from rat brain slices and on its presynaptic α-adrenergic modulation. J Neurochem 39: 349–356
Yamazaki S, Katada T, Ui M (1982) Alpha2-adrenergic inhibition of insulin secretion via interference with cyclic AMP generation in rat pancreatic islets. Mol Pharmacol 21:648–653
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Allgaier, C., Feuerstein, T.J. & Hertting, G. N-ethylmaleimide (NEM) diminishes α2-adrenoceptor mediated effects on noradrenaline release. Naunyn-Schmiedeberg's Arch. Pharmacol. 333, 104–109 (1986). https://doi.org/10.1007/BF00506511
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00506511