Abstract
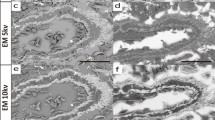
Glycosaminoglycans (GAGs) are essential components of the extracellular matrix contributing to the mechanical properties of connective tissues as well as to cell recognition and growth regulation. The ultrastructural localization of GAGs in porcine lung was studied by means of the dye Cupromeronic Blue in the presence of 0.3 M MgCl2 according to Scott's critical electrolyte concentration technique. GAGs were observed in locations described as follows. Pleura: Dermatan sulphate (DS) and chondroitin sulphate (CS) attached in the region of the d-band of collagen fibrils, interconnecting the fibrils; heparan sulphate (HS) at the surface of elastic fibers and in the basement membrane of the mesothelium and blood vessels. Bronchial cartilage: Abundant amounts of GAGs were observed in three zones: pericellular, in the intercellular matrix and at the perichondrial collagen. By enzyme digestion a superficial cartilage layer with predominantly CS could be distinguished from a deep zone with CS and keratan sulphate. The structure of the large aggregating cartilage proteoglycan was confirmed in situ. Airway epithelium: HS at the whole surface of cilia and microvilli and in the basement membrane of the epithelial cells. Alveolar wall: CS/DS at collagen fibrils, HS at the surface of elastic fibers and in the basement membranes of epithelium and endothelium.
Similar content being viewed by others
References
Anderson RGW, Hein CE (1977) Distribution of anionic sites on the oviduct ciliary membrane. J Cell Biol 72:482–492
Corrin B, Addis BJ (1990) Histopathology of the pleura. Respiration 57:160–175
Dziewiatkowski DD, La Valley J, Beaudoin AG (1989) Age-related changes in the composition of proteoglycans in sheep cartilages. Connect Tissue Res 19:103–120
Fisher LW (1993) Decorin. In: Kreis T, Vale R (eds) Guidebook to the extracellular matrix and adhesion proteins. Oxford University Press, pp 48–49
Fransson LA (1987) Structure and function of cell-associated proteoglycans. TIBS 12:406–411
Gail DB, Lenfant CJM (1983) Cells of the lung: biology and clinical implications. Am Rev Respir Dis 127:366–387
Gallagher JT, Lyon M, Steward WP (1986) Structure and function of heparan sulfate proteoglycans. Biochem J 236:313–325
Hascall GK (1980) Cartilage proteoglycans: comparison of sectioned and spread whole molecules. J Ultrastruct Res 70:369–375
Höök M, Kjellen L, Johansson S, Robinson J (1984) Cell-surface glycosaminoglycans. Ann Rev Biochem 53:847–869
Inerot S, Heinegard D (1983) Bovine tracheal cartilage proteoglycans. Variations in structure and composition with age. Coll Relat Res 3:245–262
Inoue S, Leblond CP (1988) Three-dimensional network of cords: the main component of basement membranes. Am J Anat 181:341–358
Kanwar YS, Linker A, Farquhar MG (1980) Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol 86:688–693
Karlinsky JB (1982) Glycosaminoglycans in emphysematous and fibrotic hamster lungs. Am Rev Respir Dis 125:85–88
Laurie GW, Bing JT, Kleinman HK, Hassell JR, Aumailley M, Martin GR, Feldmann RJ (1986) Localization of binding sites for laminin, heparan sulfate proteoglycan and fibronectin on basement membrane (type IV) collagen. J Mol Biol 189:205–216
Lin CQ, Bissell MJ (1993) Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J 7:737–743
Matthay MA, Landolt CC, Staub NC (1982) Differential liquid and protein clearance from the alveoli of anesthetized sheep. J Appl Physiol 53:96–104
Mecham RP (1993) Elastin. In: Kreis T, Vale R (eds) Guidebook to the extracellular matrix and adhesion proteins. Oxford University Press, pp 50–52
Moller PC, Chang JP, Partridge LR (1981) The distribution of cationized ferritin receptors on ciliated epithelial cells of rat trachea. Tissue Cell 13:731–737
Morgenroth K, Bolz J (1985) Morphological features of the interaction between mucus and surfactant on the bronchial mucosa. Respiration 47:225–231
Motomiya M, Arai H, Sato H, Yokosawa A, Nagai H, Konno K (1975) Increase of dermatan sulfate in a case of pulmoary fibrosis. Tohoku J Exp Med 115:361–365
Muir H (1983) Proteoglycans as organizers of the intercellular matrix. Biochem Soc Trans 11:613–622
Nakajima M, Irimura T, Di Ferrante D, Di Ferrante N, Nicolson GL (1983) Heparan sulphate degradation: relation to tumor invasive and metastatic properties of mouse B16 melanoma sublines. Science 220:611–613
Pasquali-Ronchetti I, Baccarani-Contri M, Fornieri C, Mori G, Quaglino JrD (1993) Structure and composition of the elastin fibre in normal and pathological conditions. Micron 24:75–89
Poole AR (1986) Proteoglycans in health and disease: structure and functions. Biochem J 236:1–14
Radhakrishnamurthy B, Ruiz HA, Berenson GS (1977) Isolation and characterization of proteoglycans from bovine aorta. J Biol Chem 252:4831–4841
Ruoslahti E (1988) Structure and biology of proteoglycans. Annu Rev Cell Biol 4:229–255
Sannes PL (1986) Cytochemical visualization of anions in collagenous and elastic fiber-associated connective tissue matrix in neonatal and adult rat lung using iron-containing stains. Histochemistry 84:49–56
Schumacher U, Barth J, Petermann W, Welsch U (1989) Ätiologie und Pathogenese von Lungenfibrosen — ein kurzer Überblick. Dtsch Ärztebl 86:C 1394–1396
Scott JE (1973) Affinity, competition and specific interactions in the biochemistry and histochemistry of polyelectrolytes. Biochem Soc Trans 1:787–806
Scott JE (1985) Proteoglycan histochemistry. A valuable tool for connective tissue biochemists. Coll Relat Res 5:541–575
Scott JE (1988) Proteoglycan-fibrillar collagen interactions. Biochem J 252:313–323
Scott JE (1992a) Morphometry of Cupromeronic Blue-stained proteoglycan molecules in animal corneas, versus that of purified proteoglycans stained in vitro, implies that tertiary structures contribute to corneal ultrastructure. J Anat 180:155–164
Scott JE (1992b) Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J 6:2639–2645
Scott JE, Haigh M (1988a) Identification of specific binding sites for keratan sulfate proteoglycans and chondroitin-dermatan sulfate proteoglycans on collagen fibrils in cornea by the use of Cupromeronic Blue in ‘critical-electrolyte-concentration’ techniques. Biochem J 253:607–610
Scott JE, Haigh M (1988b) Keratan sulphate and the ultrastructure of cornea and cartilage: a ‘stand in’ for chondroitin sulphate in conditions of oxygen lack? J Anat 158:95–108
Sleigh MA, Blahe JR, Liron N (1988) The propulsion of mucus by cilia. Am Rev Respir Dis 137:726–741
Stockwell RA, Scott JE (1967) Distribution of acid glycosaminoglycans in human articular cartilage. Nature 215:1367–1378
Takusagawa K, Ariji F, Shidda K, Sato T, Asoo N, Konno K. (1982) Electron microscopic observations on pulmonary connective tissue stained by Ruthenium Res. Histochem J 14:257–271
Vaccaro CA, Brody JS (1979) Ultrastructural localisation and characterisation of proteoglycans in the pulmonary alveolus. Am Rev Respir Dis 120:901–910
van Kuppevelt THMSM, Domen JGW, Cremers FPM, Kuyper CMA (1984a) Staining of proteoglycans in mouse lung alveoli.I. Ultrastructural localization of anionic sties. Histochem J 16:657–669
van Kuppevelt THMSM, Cremers FPM, Domen JGW, Kuyper CMA (1984b) Staining of proteoglycans in mouse lung alveoli. II. Characterisation of the Cuprolinic Blue-positive, anionic sites. Histochem J 16:671–686
van Kuppevelt THMSM, Cremers FPM, Domen JGW, van Beuningen AM, van den Brule AJC, Kuyper CMA (1985a) Ultrastructural localization and characterization of proteoglycans in human lung alveoli. Eur J Cell Biol 36:74–80
van Kuppevelt THMSM, van Beuningen AM, Rutten TLM, van den Brule AJC, Kuyper CMA (1985b) Further characterization of a large proteoglycan in human lung alveoli. Eur J Cell Biol 39:386–390
Völker W, Schmidt A, Buddecke E (1986) Compartmentation and characterization of different proteoglycans in bovine arterial wall. J Histochem Cytochem 34:1293–1299
Völker W, Schmidt A, Buddecke E (1987) Mapping of proteoglycans in human arterial tissue. Eur J Cell Biol 45:72–79
Vogel KG, Paulsson M, Heinegard D (1984) Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J 223:587–597
Winterbourne DJ, Salisbury JG (1981) Heparan sulphate is a potent inhibitor of DNA synthesis in vitro. Biochem Biophys Res Commun 101:30–37
Yanagishita M (1993) Function of proteoglycans in the extracellular matrix. Acta Pathol Jpn 43:283–293
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Erlinger, R. Glycosaminoglycans in porcine lung: an ultrastructural study using Cupromeronic Blue. Cell Tissue Res. 281, 473–483 (1995). https://doi.org/10.1007/BF00417864
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00417864