Abstract
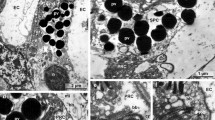
The cerebrally innervated eyes of the veliger larvae of Smaragdia sp. and Strombus sp. are composed of a lens, a cornea, and an everse retina. The retina contains two different types of cells, ciliary sensory cells and supportive cells which bear one or two cilia. It is suggested that: (a) the ciliary photoreceptors of these teleplanic veliger larvae are correlated with a long pelagic life in the ocean, which can last up to twelve months, and (b) that structural details of the photoreceptors can change during ontogenesis (ciliary vs rhabdomeric). Furthermore, the cilia of the supportive cells apparently tranport lens material and thus play an important role in lens formation. A decomposition mechanism of pigment granules is examined.
Similar content being viewed by others
Abbreviations
- bb :
-
basal body
- bp :
-
basal plate
- c :
-
cilium
- cc :
-
corneal cell
- cm :
-
ciliary membranes
- cw :
-
ciliary whorl
- ecm :
-
extracellular matrix
- gr :
-
electron-dense granules
- l :
-
lens
- lb :
-
lamellar body
- mp :
-
membranous pieces
- mt :
-
microtubules
- mv :
-
microvilli
- n :
-
nucleus
- oc :
-
optic cavity
- on :
-
optic nerve
- pg :
-
pigment granule
- sc :
-
sensory cell
- sj :
-
septate junction
- spc :
-
supportive cell
- v :
-
vesicles
References
Bartolomaeus T (1992) Ultrastructure of the photoreceptor in the larvae of Lepidochiton cinerus (Mollusca, Polyplacophora) and Lacuna, divaricata (Mollusca Gastropoda). Microfauna Marina 7:215–236
Blest AD, Price GD, Maples J (1979) Photoreceptor membrane breakdown in the spider Dinopis: Localisation of acid phosphatases. Cell Tissue Res 199:455–472
Blumer M (1994) The ultrastructure of the eyes in the veliger larvae of Aporrhais sp. and Bittium reticulatum (Mollusca, Caenogastropoda). Zoomorphology 144:149–159
Brandenburger JL (1975) Two new kind of retinal cells in the eye of a snail, Helix aspersa. J Ultrastruct Res 50:216–230
Brandenburger JL (1977) Cytochemical localization of acid phosphatase in regenerated and dark-adapted eyes of a snail, Helix aspersa. Cell Tissue Res 184:301–313
Brandenburger JL, Eakin RM (1980) Cytochemical localisation of acid phosphatase in ocelli of seastars Patiria minimata during recycling of photoreceptoral membranes. J Exp Zool 214:127–140
Brandenburger JL, Eakin RM (1985) Cytochemical localisation of acid phosphatase in light- and dark-adapted eyes of a polychaete worm Nereis limnicola. Cell Tissue Res 242:623–628
Chia FS, Koss R (1978) Development and metamorphosis of the planktotrophic larvae of Rostanga pulchra (Mollusca, Nudibranchia). Mar Biol 46:109–119
Chia FS, Koss R (1983) Fine structure of the larval eyes of Rostanga pulchra (Mollusca, Opisthibranchia, Nudibranchia). Zoomorphology 102:1–10
Crowther RJ, Bonar DB (1980) Photoreceptor ontogeny and differentiation in Ilyanassa obsoleta. Am Zool 20:886
Eakin RM, Brandenburger JL (1967a) Differentiation in the eye of a pulmonate snail Helix aspersa. J Ultrastruct Res 18:391–421
Eakin RM, Brandenburger JL (1967b) Light-induced ultrastructural changes in the eyes of pulmonate snail, Helix aspersa. J Ultrastruct Res 21:164
Eakin RM, Brandenburger JL (1974) Ultrastructural effects of dark adaption on the eyes of a snail, Helix aspersa. J Exp Zool 187:127–133
Eakin RM, Brandenburger JL (1975) Understanding a snail's eye at a snail's pace. Am Zool 15:851–863
Eakin RM, Brandenburger JL (1982) Pinocytosis in eyes of a snail, Helix aspers. J Ultrastruct Res 80:214–229
Eakin RM, Hermans CO (1988) Eyes. Microfauna Marina 4:135–156
Eakin RM, Westfall JA, Dennis MJ (1967) Fine structure of the eye of a nudibranch mollusc Hermissenda crassicornis. J Cell Sci, 2:349–358
Eakin RM, Brandenburger JL, Barker GM (1980) Fine structure of the eye of the New Zealand slug Athroracophorus bitentaculatus. Zoomorphology 94:225–239
Gibson B (1984a) Cellular and ultrastructural features of the regenerating adult eye in the marine gastropod Ilyanassa obsoleta. J Morphol 180:145–157
Gibson B (1984b) Cellular and ultrastructural features of the adult and embryonic eye of the marine gastropod Ilyanasa obsoleta. J Morphol 180:205–220
Gillary H, Gillary E (1979) Ultrastructural features of the retina and the optic nerve of Strombus luhuanus, a marine gastropod. J Morphol 159:89–116
Howard DR, Martin GG (1984) Fine structure of the eyes of the interstitial gastropod Fartulum orcutti (Gastropoda, Prosobranchia). Zoomorphology 104:197–203
Hughes HPI (1969) A light and electron microscope study of some opisthobranch eyes. Z Zellforsch 106:79–98
Hughes HPI (1970) The larval eye of the aeloid nudibranc Trinchesia aurantia (Alder and Hancock). Z Zellforsch 109:55–63
Hughes HPI (1976) Structure and regeneration of the eye of strombid gastropod. Cell Tissue Res 171:259–271
Jacklet J, Alvarez R, Bernstein B (1972) Ultrastructure of the eye of Aplysia. J Ultrastruct Res 38:246–261
Kataoka S, Yamamoto Y (1981) Diurnal changes in the fine structure of photoreceptors in an abalone, Nordotis discus. Cell Tissue Res 218:181–189
Mayes M, Hermans C (1973) Fine structure of the eye of the prosobranch mollusc Littorina scutulata. Veliger 16:166–169
Röhlich P, Török L (1963) Die Feinstruktur des Auges der Weinbergschnecke (Helix pomatia L.). Z Zellforsch 60:348–368
Salvini-Plawen L (1980) Was ist cine Trochophora? Eine Analyse der Larventypen mariner Protostomier. Zool Jb Anat 103:389–423
Salvini-Plawen L (1982) On the polyphyletic origin of photoreceptors. In: Westfall (ed) Visual cells in evolution. Raven Press, New York, pp 137–154
Salvini-Plawen L (1988) Annelida and Mollusca — a prospectus. Microfauna Marina 4:383–396
Scheltema R (1971) The dispersal of the larvae of shoal-water benthic invertebrate species over long distance by ocean currents. In: Crisp DJ (ed) Fourth European marine biology symposium. Cambridge University Press, Cambridge, pp 7–28
Scheltema R (1989) Planktonic and non-planktonic development among prosobranch gastropods and its relationship, to the geographic range of species. In: Ryland J, Tyler P (eds) Reproduction, genetics and distribution of marine organisms, 23rd European marine biology symposium. Olsen and Olsen, Fredensgorg, Denmark, pp 183–188
Tonosaki A (1967) The fine structure of the retina of Haliotis discus. Z Zellforsch 79:469–480
Waterman TH (1982) Fine structure and turnover of photoreceptor membranes. In: Westfall J (ed) Visual cells in evolution, Raven Press, New York, pp 23–41
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blumer, M.J.F. The ciliary photoreceptor in the teleplanic veliger larvae of Smaragdia sp. and Strombus sp. (Mollusca, Gastropoda). Zoomorphology 115, 73–81 (1995). https://doi.org/10.1007/BF00403256
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00403256