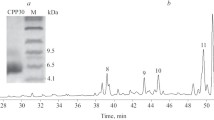
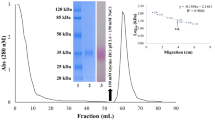
Abstract
Endopeptidase activity in mature jackbeans has been characterised. One major activity is present, that of a neutral metallo-endopeptidase. Using specific inhibitors it can be shown that the Concanavalin A-associated fragments are not formed by proteolytic degradation of the intact subunit on hydration of the tissue. The intact subunit of the lectin is also resistant to the degradation by the endogenous endopeptidase activity during a prolonged incubation in vitro. These results suggest that the lectin fragments have been formed during seed maturation and no further limited proteolysis of Concanavalin A occurs during the early stages of germination.
Similar content being viewed by others
Abbreviations
- Con A:
-
Concanavalin A
- Hepes:
-
4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid
- PAGE:
-
polyacrylamide-gel electrophoresis
- SDS:
-
sodium dodecyl sulphate
- TCA:
-
trichloroacetic acid
References
Alpi, A., Beevers, H. (1981) Proteinases and enzyme stability in crude extracts of castor bean endosperm. Plant Physiol. 67, 499–502
Barrett, A.J. (1977) Proteinases in mammalian cells and tissues. In: Research monographs in cell and tissue physiology, Dingle, J.T., ed. North-Holland Biomedical Press, Amsterdam
Baumgartner, B., Chrispeels, M.J. (1977) Purification and characterisation of vicilin peptidohydrolase, the major endopeptidase of mung bean seedlings. Eur. J. Biochem. 77, 223–233
Bond, H., Bowles, D.J. (1983) Characterisation of soybean endopeptidase activity using exogenous and endogenous substrates. Plant Physiol. (in press)
Bowles, D.J., Marcus, S. (1981) Characterisation of receptors for the endogenous lectins of soybean and jackbean seeds. FEBS Lett. 129, 135–138
Bowles, D.J., Chaplin, M.C., Marcus, S. (1983) An interaction between jackbean α-mannosidase and concanavalin A. Eur. J. Biochem. 130, 613–618
Cunningham, B.A., Wang, J.L., Waxdal, M.J., Edelman, G.M. (1975) The covalent and three dimensional structure of concanavalin A. J. Biol. Chem. 250, 1503–1512
Dalkin, K., Bowles, D.J. (1983) Analysis of inter-relationship of jackbean seed components by two-dimensional mapping of iodinated tryptic peptides. Planta 157, 536–539
George, S.G., Kenny, A.J. (1973) Studies on the enzymology of purified preparations of brush border from rabbit kidney. Biochem. J. 134, 43–57
Hopp, T.P., Hemperly, J.J., Cunningham, B.A. (1982) Amino acid sequence and varrant forms of favin, a lectin from Vicia Faba. J. Biol. Chem. 257, 4473–4483
Lotan, R., Lis, H., Sharon, N. (1975) Aggregation and fragmentation of soybean agglutinin. Biochem. Biophys. Res. Commun. 62, 114–150
Sedmark, J.J., Grossberg, S.E. (1977) A rapid sensitive and versatile assay for protein using Coomassie brilliant blue. Anal. Biochem. 79, 544–552
Tully, R.E., Beevers, H. (1978) Proteases and peptidases of castor bean endosperm. Plant Physiol. 62, 746–750
Wang, J.L., Cunningham, B.A., Edelman, G.M. (1971) Unusual fragments in the subunit structure of concanavalin A. Proc. Natl. Acad. Sci. USA 68, 1130–1134
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dalkin, K., Marcus, S. & Bowles, D.J. Endopeptidase activity in jackbeans and its effect on Concanavalin A. Planta 157, 531–535 (1983). https://doi.org/10.1007/BF00396884
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00396884