Abstract
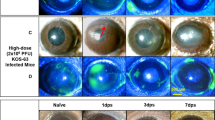
The virological, clinical, and histopathological manifestations of acute and experimentally reactivated infections of eyes and trigeminal ganglia have been studied following intranasal infection of rabbits with herpes simplex virus type 1 (strain KOS-63). All animals shed virus in nasal secretions, but only three shed virus in tear film during the first 12 days of infection. No animal developed clinical or histological evidence of corneal or retinal ocular disease at any time after infection. KOS-63 established trigeminal ganglionic latency; viral RNA, restricted to neuronal nuclei, was detected by in situ hybridization, and virus was recovered from co-cultivation cultures of nervous tissue, but not from cell-free homogenates. Reactivation of latent trigeminal ganglionic infection was attempted by intravenous administration of cyclophosphamide, followed by dexamethasone 24 h later. Injection of the drugs failed to reactivate KOS-63 latency; no animal shed virus in nasal or ocular secretions, and no animal developed gross or microscopic corneal lesions. In addition, viral antigens were not detected by immunofluorescence microscopy in ganglia from rabbits subjected to the drug protocol, and virus was only recovered from ganglia by in vitro co-cultivation reactivation techniques. The failure of KOS-63 to reactivate was not due to an inherent failure of populate and infect the ganglion, because the virus did not reactivate from ganglia that contained many latently infected cells. These studies demonstrate that, although KOS-63 is neuroinvasive and capable of establishing latency, it is virtually non-virulent for the eye, and cannot be reactivated by a systemic immunosuppressive trigger known to reactivate other HSV-1 strains.
Similar content being viewed by others
References
Arao Y, Hatano A, Yamada M, Uno F, Nii S (1991) Reactivatable latency of three avirulent strains of herpes simplex virus type 1 after intranasal inoculation of mice. Acta Med Okayama 45: 43–47
Batra SK, Brown SM (1990) Herpes simplex virus genes controlling reactivation from latency in rabbit eye model. Indian J Med Res 91: 252–257
Block TM, Spivack JG, Steiner I, Deshmane S, McIntosh MT, Lirette RP, Fraser NW (1990) A herpes simplex virus type 1 latency-associated transcript mutant reactivates with normal kinetics from latent infection. J Virol 64: 3417–3426
Brandt CR, Kitner RL, Pumfery AM, Visalli RJ, Grau DR (1991) The herpes simplex virus ribonucleotide reductase is required for ocular virulence. J Gen Virol 72: 2043–2050
Centifanto-Fitzgerald YM, Rayfield M, Tian P-Y, Kaufman HE (1985) Herpex simplex virus latency in the rabbit trigeminal ganglia: ganglionic superinfection. Proc Soc Exp Biol Med 179: 55–67
Centifanto-Fitzgerald Y, Caldwell DR, Yates F (1987) Herpex simplex virus: recurrent and nonrecurrent strains. Proc Soc Exp Biol Med 185: 484–492
Corey L, Spear PG (1986) Infection with herpex simplex viruses. N Engl J Med 314: 749–757
Culbertson WW, Blumenkrantz MS, Haines H, Gass DM, Mitchell KB, Norton EWD (1982) The acute retinal necrosis syndrome. Ophthalmology 89: 1317–1325
Day SP, Lausch RN, Oakes JE (1987) Nucleotide sequences important in DNA replication are responsible for differences in the capacity of two herpes simplex virus strains to spread from cornea to central nervous system. Curr Eye Res 6: 19–26
Dix RD, McKendall RR, Baringer JR (1983) Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun 840: 103–112
Goldin AL, Sandri-Goldin RM, Levine M, Glorioso JC (1981) Cloning of herpes simplex virus type 1 sequences representing the whole genome. J Virol 38: 50–58
Gordon YJ, Romanowski E, Balouris C, Araullo-Cruz T (1990) A herpes simplex type 1 ICP0 mutant demonstrates diminished pathogenicity during acute ocular infection in different host animals. Invest Ophthalmol Vis Sci 31: 681–688
Grau DR, Visalli RJ, Brandt CR (1989) Herpes simplex virus stromal keratitis is not titer-dependent and does not correlate with neurovirulence. Invest Ophthalmol Vis Sci 30: 2472–2480
Hill JM, Rayfield MA, Haruta Y (1987) Strain specificity of spontaneous and adrenergically induced HSV-1 ocular reactivation in latently infected rabbits. Curr Eye Res 6: 91–97
Ho DY, Mocarski ES (1989) Herpes simplex virus latent RNA (LAT) is not required for latent infection in the mouse. Proc Natl Acad Sci USA 86: 7596–7600
Javier RT, Stevens JG, Dissette VB, Wagner EK (1988) A herpes simplex virus transcript abundant in latently infected neurons is dispensable for establishment of the latent state. Virology 166: 254–257
Kaufman HE (1978) Herpetic keratitis. Invest Ophthalmol Vis Sci 17: 941–957
Leib DA, Bogard CL, Kosz-Vnenchak M, Hicks KA, Coen DM, Knipe DM, Schaffer PA (1989) A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol 63: 2893–2900
Martin JR, Jenkins FJ, Henken DB (1991) Targets of herpes simplex virus type-1 infection in a mouse cornea model. Acta Neuropathol 82: 353–363
McKendall RR, Woo W (1988) Antibody activity to herpes simplex virus in mouse Ig classes and IgG subclasses. Arch Virol 98: 225–233
Meyers-Elliot R, Chitjian PA, Dethlefs BA (1983) Experimental herpesvirus keratitis in the rabbit: topical versus intrastromal infection routes. Ophthalmic Res 15: 240–256
Mitchell WJ, Steiner I, Brown SM, MacLean AR, Subak-Sharpe JH, Fraser NW (1990) A herpes simplex virus type 1 variant, deleted in the promoter region of the latency-associated transcripts, does not produce any detectable minor RNA species during latency in the mouse trigeminal ganglion. J Gen Virol 71: 953–958
Pavan-Langston DR (1984) Ocular viral diagnosis. In: Galasso GJ, Merigan TC, Buchanen RA (eds) Antiviral agents and viral diseases of man, 2nd edn Raven Press, New York, pp 207–246
Pavan-Langston D, Grene B (1984) Herpes simplex ocular disease. Compr Ther: 30–36
Romanowski EG, Araullo-Cruz T, Gordon YJ (1993) Viral genetic differences appear to play a role in the establishment of latency for different wt viruses in the NZ rabbit eye model. Invest Ophthalmol Vis Sci 34: 1345
Rootman DS, Haruta Y, Hill JM, Kaufman HE (1988) Corneal nerves are necessary for adrenergic reactivation of ocular herpes. Invest Ophthalmol Vis Sci 29: 351–392
Shimomura Y, Gangarosa LP, Kataoka M, Hill JM (1983) HSV-1 shedding by iontophoresis of 6-hydroxydopamine followed by topical epinephrine. Invest Ophthalmol 24: 1588–1594
Shimomura Y, Dudley JB, Gangarosa LP, Hill JM (1985) HSV-1 quantitation from rabbit neural tissues after epinephrine-induced reactivation. Invest Ophthalmol Vis Sci 26: 121–125
Steiner I, Spivack JG, Lirette RP, Brown SM, MacLean AR, Subak-Sharpe JH, Fraser NW (1989) Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J 8: 505–511
Stevens JG, Wagner EK, Devi-Rao GB, Cook ML, Feldman LT (1987) RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235: 1056–1059
Strauss SE, Rooney JF, Sever JL, Seidlin M, Nusinoff-Lehrman S, Cremer K (1985) Herpes simplex virus infection: biology, treatment and prevention. Ann Intern Med 103: 404–419
Stroop WG (1986) Herpes simplex virus encephalitis of the human adult: reactivation of latent brain infection. Pathol Immunopathol Res 5: 156–169
Stroop WG, Schaefer DC (1986) Experimental reactivation of herpex simplex virus produces encephalitis restricted to the temporal lobes. J Infect Dis 153: 721–731
Stroop WG, Schaefer DC (1987) Severity of experimentally reactivated herpetic eye disease is related to the neurovirulence of the latent virus. Invest Ophthalmol Vis Sci 28: 229–237
Stroop WG, Schaefer DC (1987) Herpes simplex virus, type 1 invasion of the rabbit and mouse nervous system revealed by in situ hybridization. Acta Neuropathol (Berl) 74: 124–132
Stroop WG, Schaefer DC (1989) Neurovirulence of two clonally related herpes simplex virus type 1 strains in a rabbit seizure model. J Neuropathol Exp Neurol 48: 171–183
Stroop WG, Rock DL, Fraser NW (1984) Localization of herpes simplex virus in the trigeminal and olfactory systems of the mouse central nervous system during acute and latent infections by in situ hybridization. Lab Invest 51: 27–38
Stroop WG, McKendall RR, Battles EJ, Schaefer DC, Jones B (1990) Spread of herpes simplex virus type 1 in the central nervous system during experimentally reactivated encephalitis. Microb Pathog 8: 119–134
Thompson RL, Stevens JG (1983) Biological characteristics of a herpes simplex virus intertypic recombinant which is completely and specifically nonneurovirulent. Virology 131: 171–179
Wagner EK, Flanagan WM, Devi-Rao G, Zhang Y-F, Hill JM, Anderson KP, Stevens JG (1988) The herpes simplex virus latency-associated transcript is spliced during the latent phase of infection. J Virol 62: 4577–4585
Wander AH, Centifanto YM, Kaufman HE (1980) Strain specificity of clinical isolates of herpes simplex virus. Arch Ophthalmol 98: 1458–1461
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stroop, W.G., Banks, M.C. Herpes simplex virus type 1 strain KOS-63 does not cause acute or recurrent ocular disease and does not reactivate ganglionic latency in vivo. Acta Neuropathol 87, 14–22 (1994). https://doi.org/10.1007/BF00386250
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00386250