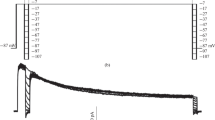
Abstract
The whole-cell configuration of the patch-clamp technique was used to examine K+ currents in HeLa cells. Under quasi-physiological ionic gradients, using an intracellular solution containing 10−7 mol/l free Ca2+, mainly outward currents were observed. Large inwardly rectifying currents were elicited in symmetrical 145 mmol/l KCl. Replacement of all extracellular K+ by isomolar Na+, greatly decreased inward currents and shifted the reversal potential as expected for K+ selectivity. The inwardly rectifying K+ currents exhibited little or no apparent voltage dependence within the range of from −120 mV to 120 mV. A square-root relationship between chord conductance and [K+]0 at negative potentials could be established. The inwardly rectifying nature of the currents was unaltered after removal of intracellular Mg2+ and chelation with ATP and ethylenediaminetetraacetic acid (EDTA). Permeability ratios for other monovalent cations relative to K+ were: K+ (1.0)>Rb+ (0.86)>Cs+ (0.12)>Li+ (0.08)>Na+ (0.03). Slope conductance ratios measured at −100 mV were: Rb+ (1.66)>K+ (1.0)>Na+ (0.09)>Li+ (0.08)>Cs+ (0.06). K+ conductance was highly sensitive to intracellular free Ca2+ concentration. The relationship between conductance at 0 mV and Ca2+ concentration was well described by a Hill expression with a dissociation constant, K D, of 70 nmol/l and a Hill coefficient, n, of 1.81. Extracellular Ba2+ blocked the currents in a concentration- and voltage-dependent manner. The dependence of the K D for the blockade was analysed using a Woodhull-type treatment, locating the ion interaction site at 19 % of the distance across the electrical field of the membrane and a K D (0 mV) of 7 mmol/l. Tetraethylammonium and 4-aminopyridine were without effect whilst quinine and quinidine blocked the currents with concentrations for half-maximum effects equal to 7 μmol/l and 3.5 μmol/l, respectively. The unfractionated venom of the scorpion Leiurus quinquestriatus (LQV) blocked the K+ currents of HeLa cells. The toxins apamin and scyllatoxin had no detectable effect whilst charybdotoxin, a component of LQV, blocked in a voltage-dependent manner with half-maximal concentrations of 40 nmol/l at −120 mV and 189 nmol/l at 60 mV; blockade by charybdotoxin accounts for the effect of LQV. Application of ionomycin (5–10 μmol/l), histamine (1 mmol/l) or bradykinin (1–10 μmol/l) to cells dialysed with low-buffered intracellular solutions induced K+ currents showing inward rectification and a lack of voltage dependence.
Similar content being viewed by others
References
Adams DJ, Barakeh J, Lakey R, van Breemen C (1989) Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J 3:2389–2400
Anderson C, Mackinnon R, Smith C, Miller C (1988) Charybdotoxin inhibition of Ca2+-activated K+ channels. Effects of channel gating, voltage and ionic strength. J Gen Physiol 91:317–333
Ashcroft FM (1988) Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci 11:97–118
Banderali U, Roy G (1992) Activation of K+ and Cl− channels in MDCK cells during volume regulation in hypotonic media. J Membr Biol 126:219–234
Blatz AL, Magleby KL (1984) Ion conductance and selectivity of single calcium-activated potassium channels in cultured rat muscle. J Gen Physiol 84:1–23
Castle NA, Haylett DG, Jenkinson DH (1989) Toxins in the characterization of potassium channels. Trends Neurosci 12:59–65
Christensen O, Hoffmann EK (1992) Cell swelling activates K+ and Cl− channels as well as nonselective, stretch-activated cation channels in Ehrlich ascites tumor cells. J Membr Biol 129:13–36
Colden-Stanfield M, Schilling WP, Possani LD, Kunza DL (1990) Bradykinin-induced potassium current in cultured bovine aortic endothelial cells. J Membr Biol 146:227–238
Devor DC, Frizzell RA (1993) Calcium-mediated agonists activate an inwardly rectified K+ channel in colonic secretory cells. Am J Physiol 265:C1271-C1280
Díaz M, Valverde MA, Higgins CF, Rucareaunu C, Sepúlveda FV (1993) Volume-activated chloride channels in HeLa cells are blocked by verapamil and dideoxyforskolin. Pflügers Arch 422:347–353
Eisenman G, Horn R (1983) Ionic selectivity revisited: the role of kinetic and equilibria process in ion permeation through channels. J Membr Biol 76:197–225
Findlay I (1987) The effects of magnesium upon adenosine triphosphate-sensitive potassium channels in a rat insulin-secreting cell line. J Physiol (Lond) 391:611–629
Findlay I, Dunne MJ, Ullrich S, Wollheim CB, Petersen OH (1985) Quinine inhibits Ca2+-independent K+ channels whereas tetraethylammonium inhibits Ca2+-activated K+ channels in insulin-secreting cells. FEBS Lett 185:4–8
Fukushima Y (1982) Blocking kinetics of the anomalous potassium rectifier of the tunicate egg studied by single channel recording. J Physiol (Lond) 331:311–331
Gallin EK (1989) Evidence for Ca-activated inwardly-rectifying K channel in human macrophages. Am J Physiol 257:C77-C85
Garcia ML, Gálvez A, García-Calvo M, King VF, Vázquez J, Kaczorowski GJ (1991) Use of toxins to study potassium channels. J Bioeng Biomembr 23:615–646
Gay LA, Stanfield PR (1978) The selectivity of the delayed potassium conductance of frog skeletal muscle fibers. Pflügers Arch 378:177–179
Gögelein H, Capek K (1990) Quinine inhibits chloride and non-selective cation channels in isolated rat distal colon cells. Biochim Biophys Acta 1027:191–198
Gögelein H, Greger R, Schlatter E (1987) Potassium channels in the basolateral membrane of the rectal gland of Squalus acanthias. Regulation and inhibitors. Pflügers Arch 409:107–113
Grissmer S, Nguyen AN, Cahalan MD (1993) Calcium-activated potassium channels in resting and activated human T lymphocytes. J Gen Physiol 102:601–630
Grygorczyk R, Schwarz W, Passow H (1984) Ca2+-activated K+ channels in human red cells. Comparison of single-channel currents with ion fluxes. Biophys J 45:693–698
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth J (1981) Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Harvey RD, Ten Eick RE (1988) Characterization of the inward-rectifying potassium current in cat ventricular myocytes. J Gen Physiol 91:593–615
Haylett DG, Jenkinson DH (1990) Calcium-activated potassium channels. In: Nigel S. Cook (ed) Potassium channels, structure, classification, function and therapeutic potential. Ellis Horwood, Chichester, pp 70–95
Hazama A, Okada Y (1985) HeLa cells have histamine H1-receptors which mediate activation of the K+ conductance. Biochim Biophys Acta 845:249–253
Hille B (1992) Ionic channels of excitable membranes. Sinauer Associates, Sunderland, Mass.
Huang Y, Rane SG (1993) Single channel study of a Ca2+-activated K+ current associated with ras-induced cell transformation. J Physiol (Lond) 461:601–618
Ishikawa T, Cook DI (1993) Effects of K+ channel blockers on inwardly and outwardly rectifying whole-cell K+ currents in sheep parotid secretory cells. Pflügers Arch 133:29–41
Iwatsuki N, Petersen OH (1985) Action of tetraethylammonium on calcium-activated potassium channels in pig pancreatic acinar cells studied by patch-clamp single-channel and whole-cell current recording. J Membr Biol 86:139–144
Lang F, Paulmichl M, Pfeilschifter J, Friedrich F, Woll E, Waldegger S, Ritter M, Tschernko E (1991) Cellular mechanisms of bradykinin-induced hyperpolarization in renal epitheloid MDCK-cells. Biochim Biophys Acta 1073:600–608
Latorre R, Miller C (1983) Conduction and selectivity in potassium channels. J Membr Biol 71:11–30
MacKinnon R, Miller C (1988) Mechanism of charybdotoxin block of Ca2+-activated K+ channels. J Gen Physiol 91:335–349
Matsuda H (1991) Magnesium gating of the inwardly rectifying K+ channel. Annu Rev Physiol 53:289–298
Moczydlowski E, Lucchesi K, Ravindran A (1988) An emerging pharmacology of peptide toxins targeted against potassium channels. J Membr Biol 105:95–111
Paradiso A, Chang EHC, Boucher RC (1991) Effects of bradykinin in human ciliated airway epithelia. Am J Physiol 261:L63-L69
Pavenstädt H, Bengen F, Spath M, Schollmeyer P, Greger R (1993) Effect of bradykinin and histamine on the membrane voltage, ion conductances and ion channels of human glomerular epithelial cells (hGEC) in culture. Pflügers Arch 424:137–144
Pfaffinger PJ, Siegelbaum SA (1990) K+ channel modulation by G protein and second messengers. In: Cook N. S. (ed) Potassium channels, structure, classification, function and therapeutic potential. Ellis Horwood, Chichester, pp 117–141
Rorsman P, Trube G (1990) Biophysics and physiology of ATP-regulated K+ channels. In: Cook N. S. (ed) Potassium channels, structure, classification, function and therapeutic potential. Ellis Horwood, Chichester, pp 96–116
Sauvé R, Roy G, Payet D (1983) Single channel K+ currents from HeLa cells. J Membr Biol 74:41–49
Sauvé R, Simoneau C, Monette R, Roy G (1986) Single-channel analysis of the potassium permeability in HeLa cancer cells: evidence for a calcium-activated potassium channel of small unitary conductance. J Membr Biol 92:269–282
Sauvé R, Simoneau C, Parent L, Monette R, Roy G (1987) Oscillatory activation of calcium-dependent potassium channels in HeLa cells induced by histamine H1 receptor stimulation: a single-channel study. J Membr Biol 96:199–208
Sepúlveda FV, Fargon F, McNaughton PA (1991) K+ and Cl− currents in enterocytes isolated from guinea-pig small intestinal villi. J Physiol (Lond) 434:351–367
Strong PN (1990) Potassium channel toxins. Pharmacol Ther 46:137–162
Takahashi A, Yamaguchi H, Miyamoto H (1993) Change in K+ current of HeLa cells with progression of the cell cycle studied by patch-clamp technique. Am J Physiol 265:C328-C336
Taylor PS (1987) Selectivity and patch measurements of A-currents in Helix aspersa neurons. J Physiol (Lond) 388:437–447
Thuringer D, Diarra A, Sauvé R (1991) Modulation by extracellular pH of bradykinin-evoked activation of Ca2+-activated K+ channels in endothelial cells. Am J Physiol 261:H656-H666
Tilly BC, Kansen M, van Gageldonk PG, van den Berghe N, Bijman J (1990) Activation of intestinal chloride channels by GTP-binding regulatory proteins. Adv Second Messenger Phosphoprotein Res 24:95–100
Tivey DR, Simmons NL, Aiton JF (1985) Role of passive potassium fluxes in cell volume regulation in cultured HeLa cells. J Membr Biol 87:93–105
Vaca L, Schilling WP, Kunze D (1992) G-protein-mediated regulation of a Ca2+-dependent K+ channel in cultured vascular endothelial cells. Pflügers Arch 422:66–74
Valverde MA, Díaz M, Sepúlveda FV, Gill DH, Hyde SC, Higgins CF (1992) Volume-regulated chloride channels associated with the multidrug resistance P-glycoprotein. Nature 355:830–833
Vergara C, Latorre R (1983) Kinetics of Ca2+-activated K+ channels from rabbit muscle incorporated into bilayers. Evidence for Ca2+ and Ba2+ blockade. J Gen Physiol 82: 543–568
Vergara C, Moczydlowski E, Latorre R (1984) Conduction, blockade and gating in a Ca2+-activated K+ channel incorporated into planar lipid bilayers. Biophys J 456:73–76
Welsh MJ, McCann JD (1985) Intracellular calcium regulates basolateral potassium channels in a chloride secreting epithelia. Proc Natl Acad Sci USA 82:8823–8826
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Díaz, M., Sepúlveda, F.V. Characterisation of Ca2+-dependent inwardly rectifying K+ currents in HeLa cells. Pflügers Arch. 430, 168–180 (1995). https://doi.org/10.1007/BF00374647
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00374647