Summary
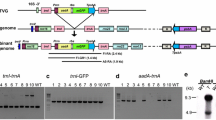
Two types of genomic DNA hybridizing with a chalcone synthase cDNA were isolated from cell suspension cultures of parsley (Petroselinum crispum cv. Mooskrause) and cloned in λEMBL4. Their fragmentation patterns with several common restriction enzymes were identical, except for the occurrence of a 927 base pair insertion in one type relative to the other. This insertion is located 538 base pairs upstream of the first of two transcription start sites and has characteristic features of a transposable element. The two types of cloned DNA most likely represent two alleles of a chalcone synthase gene occurring in one copy per haploid parsley genome. The nucleotide sequence and exon-intron structure of the larger allele were determined. Analysis of plants either heterozygous or homozygous with respect to the chalcone synthase gene revealed that both allelic forms were expressed and activated by UV light.
Similar content being viewed by others

References
Aviv H, Leder P (1972) Purification of biologically active globin mRNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci USA 69:1408–1412
Bruns B, Hahlbrock K, Schäfer E (1986) Fluence dependence of the ultraviolet-light-induced accumulation of chalcone synthase mRNA and effects of blue and far-red light in cultured parsley cells. Planta 169:393–398
Chappell J, Hahlbrock K (1984) Transcription of plant defense genes in response to UV light or fungal elicitor. Nature 311:76–78
Dangl JL, Hauffe KD, Lipphardt S, Hahlbrock K, Scheel D (1987) Parsley protoplasts retain differential responsiveness to UV light and fungal elicitor. EMBO J 9:2551–2556
Davis RW, Hyman RW (1971) A Study in evolution: The DNA base sequence homology between coliphages T7 and T3. J Mol Biol 62:287–301
Davis RW, Simon M, Davidson N (1971) Electron microscope heteroduplex methods for mapping regions of base sequence homology in nucleic acids. Methods Enzymol 21:413–428
Dixon RA (1986) The phytoalexin response: elicitation, signalling and control of host gene expression. Biol Rev 61:239–291
Douglas C, Hoffmann H, Schulz W, Hahlbrock K (1987) Structure and elicitor or UV-light stimulated expression of two 4-coumarate: CoA ligase genes in parsley. EMBO J 6:1189–1195
Ebel J, Hahlbrock K (1982) Biosynthesis. In: Harborne JB, Mabry TJ (eds) The Flavonoids. Chapman and Hall, London New York, pp 641–675
Frischauf AM, Lehrach H, Poustka A, Murray N (1983) Lambda replacement vectors carrying polylinker sequences. J Mol Biol 170:827–842
Harborne JB, Turner BL (1984) Plant Chemosystematics. Academic Press, London
Heller W, Hahlbrock K (1980) Highly purified “flavone synthase” from parsley catalyzes the formation of naringenin chalcone. Arch Biochem Biophys 200:617–619
Henikoff S (1984) Umdirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene 28:351–359
Huang PL, Hahlbrock K, Somssich IE (1988) Detection of a single-copy gene on plant chromosomes by in situ hybridization. Mol Gen Genet 211:143–147
Jahnen W, Hahlbrock K (1988) Differential regulation and tissue-specific distribution of enzymes related to phenylpropanoid branch pathways in developing parsley seedlings. Planta (in press)
Kaulen H, Schell J, Kreuzaler F (1986) Light-induced expression of the chimeric chalcone synthase-NPT II gene in tobacco cells. EMBO J 5:1–8
Kiper M (1979) Gene numbers as measured by single-copy DNA saturation with mRNA are routinely overstimated. Nature 278:279–280
Koes RE, Spelt CE, Mol JNM, Gerats AGM (1987) The chalcone synthase family of Petunia hybrida (V30): sequence homology, chromosomal localization and evolutionary aspects. Plant Mol Biol 10:159–169
Kombrink E, Hahlbrock K (1986) Responses of cultured parsley cells to elicitors from phytopathogenic fungi. Plant Physiol 81:216–221
Konarska M, Grabowski P, Padgett RA, Sharp PA (1985) Characterization of the branch site in lariat RNAs produced by splicing of mRNA precursors. Nature 313:552–557
Kreuzaler F, Hahlbrock K (1972) Enzymatic synthesis of aromatic compounds in higher plants: formation of naringenin (5,7,4′-trihydroxyflavanone) from p-coumaroyl coenzyme A and malonyl coenzyme A. FEBS Lett 28:69–72
Kreuzaler F, Hahlbrock K (1973) Flavonoid glycosides from illuminated cell suspension cultures of Petroselinum hortense. Phytochemistry 12:1149–1152
Kreuzaler F, Ragg H, Heller W, Tesch R, Witt I, Hammer D, Hahlbrock K (1979) Flavanone synthase from Petroselinum hortense. Molecular weight, subunit composition, size of messenger mRNA, and absence of pantetheinyl residue. Eur J Biolchem 99:89–96
Kreuzaler F, Ragg H, Fautz E, Kuhn DN, Hahlbrock K (1983) UV-induction of chalcone synthase mRNA in cell suspension cultures of Petroselinum hortense. Proc Natl Acad Sci USA 80:2591–2593
Kuhn DN, Chappell J, Boudet A, Hahlbrock K (1984) Induction of phenylalanine ammonia-lyase and 4-coumarate: CoA ligase in cultured plant cells by UV-light or fungal elicitor. Proc Natl Acad Sci USA 81:1102–1106
Lee DC, Roeder RG (1981) Transcription of adenovirus Types 2 genes in a cell-free system: apparent heterogenity of initiation at some promotors. Mol Cell Biol 1:635–651
Luse DS, Haynes JR, Van Leeuwen D, Schon EA, Cleary ML, Shapiro SG, Lingrel JB, Roeder RG (1981) Trancription of the β-like globin genes and pseudogenes of the goat in a cell-free system. Nucleic Acids Res 9:4339–4354
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:6323–6327
Pohlman RF, Fedoroff NV, Messing J (1984) The nucleotide sequence of the maize controlling element activator. Cell 37:635–643
Reif HJ, Niesbach U, Deumling B, Saedler H (1985) Cloning and analysis of two genes for chalcone synthase from Petunia hybrida. Mol Gen Genet 199:208–215
Reimold U, Kröger M, Kreuzaler F, Hahlbrock K (1983) Coding and 3′ non-coding nucleotide sequence of chalcone synthase mRNA and assignment of amino acid sequence of the enzyme. EMBO J 2:1801–1805
Ryder TB, Hedrick SA, Bell JN, Liang X, Clouse SD, Lamb CJ (1987) Organization and differential activation of a gene family encoding the plant defense enzyme chalcone synthase in Phaseolus vulgaris. Mol Gen Genet 210:219–233
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Scheel D, Dangl J, Douglas C, Hauffe KD, Herrmann A, Hoffmann H, Lozoya E, Schulz W, Hahlbrock K (1987) In: von Wettstein D (ed) Stimulation of phenylpropanoid pathways by environmental factors. NATO ASI Series, pp 315–326
Schmelzer E, Börner H, Grisebach H, Ebel J, Hahlbrock K (1984) Phytoalexin synthesis in soybean (Glycine max). Similar time courses of mRNA induction in hypocotyls infected with a fungal pathogen and in cell cultures treated with fungal elicitor. FEBS Lett 172:59–63
Schmelzer E, Jahnen W, Hahlbrock K (1988) In situ localization of ligh-induced chalcone synthase mRNA, chalcone synthase, and flavonoid end products in epidermal cells of parsley leaves. Proc Natl Acad Sci USA (in press)
Schröder J, Kreuzaler F, Schäfer E, Hahlbrock K (1979) Concomitant induction of phenylalanine ammonia-lyase and flavone synthase mRNAs in irradiated plant cells. J Biol Chem 254:57–65
Schwarz-Sommer Zs (1987) The significance of plant transposable elements in biological processes. In: Henning W (ed) Results and problems of cell differentiation 14; Structure and function of eukaryotic chromosomes; Springer, Berlin Heidelberg, pp 213–221
Serfling E, Jasin M, Schaffner W (1985) Enhancers and eukaryotic gene transcription. Trends Genet 1:224–230
Sommer H, Saedler H (1986) Structure of the chalcone synthase gene of Antirrhinum majus. Mol Gen Genet 202:429–434
Sommer H, Carpenter R, Harrison BJ, Saedler H (1985) The transposable element Tam3 of Antirrhinum majus generates a novel type of sequence alterations upon excision. Mol Gen Genet 199:225–231
Weaver RF, Weissmann C (1979) Mapping of RNA by a modification of the Berk-Sharp procedure: the 5′ termini of 15 S β-globin mRNA precursor and mature 10S β-globin mRNA have identical map coordinates. Nucleic Acids Res 7:1175–1191
Wienand U, Weydemann U, Niesbach-Klösgen U, Peterson PA, Saedler H (1986) Molecular cloning of the c2 locus of Zea mays, the gene coding for chalcone synthase. Mol Gen Genet 203:202–207
Wiermann R (1981) Secondary plant products and cell and tissue differentiation. In: Stumpf PK, Conn EE (eds) The biochemistry of plants, vol. 7. Academic Press, New York London Toronto Sydney San Francisco, pp 85–112
Yanish-Perron C, Vieira J, Messing J (1985) Improved M13 cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–109
Author information
Authors and Affiliations
Additional information
Communicated by H. Saedler
Rights and permissions
About this article
Cite this article
Hermann, A., Schulz, W. & Hahlbrock, K. Two alleles of the single-copy chalcone synthase gene in parsley differ by a transposon-like element. Molec. Gen. Genet. 212, 93–98 (1988). https://doi.org/10.1007/BF00322449
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00322449