Summary
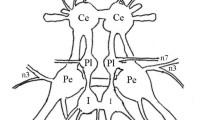
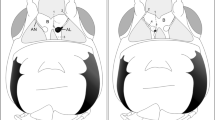
The distribution of octopamine-immunoreactive neurons is described using whole-mount preparations of all central ganglia of the cricket, Gryllus bimaculatus. Up to 160 octopamine-immunoreactive somata were mapped per animal. Medial unpaired octopamine-immunoreactive neurons occur in all but the cerebral ganglia and show segment-specific differences in number. The position and form of these cells are in accordance with well-known, segmentally-organized clusters of large dorsal and ventral unpaired medial neurons demonstrated by other techniques. In addition, bilaterally arranged groups of immunoreactive somata have been labelled in the cerebral, suboesophageal and terminal ganglia. A detailed histological description of octopamine-immunoreactive elements in the prothoracic ganglion is given. Octopamine-immunoreactive somata and axons correspond to the different dorsal unpaired medial cell types identified by intracellular single-cell staining. In the prothoracic ganglion, all efferent neurons whose primary neurites are found in the fibre bundle of dorsal unpaired cells are immunoreactive. Intersegmental octopamine-immunoreactive neurons are also present. Collaterals originating from dorsal intersegmental fibres terminate in different neuropils and fibre tracts. Fine varicose fibres have been located in several fibre tracts, motor and sensory neuropils. Peripheral varicose octopamine-immunoreactive fibres found on several nerves are discussed in terms of possible neurohemal releasing sites for octopamine.
Similar content being viewed by others
References
Adams ME, Bishop CA, O'Shea M (1983) Strategies for the identification of amine- and peptide-containing neurons. In: Strausfeld NJ (ed) Functional neuroanatomy. Springer, Berlin Heidelberg New York, pp 238–242
Agricola H, Eckert M, Ude J, Birkenbeil H, Penzlin H (1985) The distribution of a proctolin-like immunoreactive material in the terminal ganglion of the cockroach, Periplaneta americana L. Cell Tissue Res 239:203–209
Agricola H, Hertel W, Penzlin H (1988) Octopamine-neurotransmitter, neuromodulator, neurohormone. Zool Jb Physiol 92:1–45
Anwyl R, Finlayson LH (1973) The ultrastructure of neurons with both a motor and a neurosecretory function in the insect, Rhodnius prolixus. Z Zellforsch 146:367–374
Arikawa K, Washio H, Tanaka Y (1984) Dorsal unpaired median neurons of the cockroach metathoracic ganglion. J Neurobiol 15:531–536
Baily BA, Martin RJ, Downer GH (1983) Haemolymph octopamine levels during and following flight in the American cock-roach, Periplaneta americana L. Can J Zool 62:19–22
Bate CM (1976) Embryogenesis of an insect nervous system. I. A map of the thoracic and abdominal neuroblast in Locusta migratoria. J Embryol Exp Morphol 35:107–123
Bate M, Goodman CS, Spitzer NC (1981) Embryonic development of identified neurons: segment-specific differences in the H cell homologues. J Neurosci 1:103–106
Bishop CA, O'Shea M (1982) Neuropeptide proctolin (H-Arg-Try-Leu-Pro-Thr-OH): immunocytochemical mapping of neurons in the central nervous system of the cockroach. J Comp Neurol 207:223–238
Bräunig P (1987) The satellite nervous system—an extensive neurohemal network in the locust. J Comp Physiol [A] 160:69–77
Bräunig P (1988) Identification of a single prothoracic ‘dorsal unpaired median’ (DUM) neuron supplying locust mouthpart nerves. J Comp Physiol [A] 163:835–840
Bräunig P (1991) Suboesophageal DUM neurons innervate the principal neuropiles of the locust brain. Philos Trans R Soc Lond (Biol) 332:221–240
Bräunig P, Allgäuer C, Honegger HW (1990) Suboesophageal DUM neurones are part of the antennal motor system of locusts and crickets. Experientia 46:259–261
Breidbach O, Dircksen H (1989) Proctolin-immunoreactive neurons persist during metamorphosis of an insect: a developmental study of the ventral nerve cord of Tenebrio molitor (Coleoptera). Cell Tissue Res 257:217–225
Buijs RM, Pool CW, Van Heerikhuize JJ, Sluiter AA, Van der Sluis PJ, Ramkema M, Van der Woude TP, Van der Beek E (1989) Antibodies to small transmitter molecules and peptides: production and application of antibodies to dopamine, serotonin, gaba, vasopressin, vasoactive intestinal peptide, neuropeptide Y, somatostatin and substance P. Biomed Res 10 [Suppl 3]:213–222
Carlson AD, Jalenak M (1986) Release of octopamine from photomotor neurones of the larval firefly lanterns. J Exp Biol 122:453–457
Christensen TA, Carlson AD (1981) Symmetrically organized dorsal unpaired median DUM neurones and flash control in the male firefly, Photuris versicolor. J Exp Biol 93:133–147
Christensen TA, Carlson AD (1982) The neurophysiology of larval firefly luminescence, direct activation through four bifurcating DUM neurons. J Comp Physiol 148:503–514
Crossman AR, Kerkut GA, Walker RJ (1971) Axon pathways of electrically excitable nerve cell bodies in the insect central nervous system. J Physiol (Lond) 218:55–56
Davenport AP, Evans PD (1984) Changes in haemolymph octopamine levels associated with food deprivation in the locust, Schistocerca gregaria. Physiol Entomol 9:269–274
David JC, Coulon JF (1979) Octopamine distribution in the Locusta migratoria nervous and non-nervous system. Comp Biochem Physiol 64C:161–164
David JC, Coulon JF (1985) Octopamine in invertebrates and vertebrates, a review. Prog Neurobiol 24:141–185
David JC, Coulon JF, Lafon-Cazal M (1985) Octopamine changes in nervous and non-nervous tissues of the locust, Locusta migratoria L., after different flight conditions. Comp Biochem Physiol 82C:427–432
Davis NT (1985) Serotonin-immunoreactive nerves and neurohemal system in the cockroach Periplaneta americana (L.). Cell Tissue Res 240:593–600
Davis NT (1987) Neurosecretory neurons and their projections to the serotonin neurohemal system of the cockroach Periplaneta americana (L.) and identification of mandibular and maxillary neurons associated with this system. J Comp Neurol 259:604–621
Davis NT, Velleman SG, Kingan TG, Keshishian H (1989) Identification and distribution of a proctolin-like neuropeptide in the nervous system of the gypsy moth, Lymantria dispar, and other Lepidoptera. J Comp Neurol 283:71–85
Dircksen H, Müller A, Keller R (1991) Crustacean cardioactive peptide in the nervous system of the locust, Locusta migratoria. An immunohistochemical study on the ventral nerve cord and peripheral innervation. Cell Tissue Res 263:439–457
Duve H, Thorpe A, Nässel DR (1988) Light- and electron-microscopic immunocytochemistry of peptidergic neurons innervating thoracico-abdominal neurohaemal areas in the blowfly. Cell Tissue Res 253:583–595
Dymond GR, Evans PD (1979) Biogenic amines in the nervous system of the cockroach, Periplaneta americana: association of octopamine with mushroom bodies and dorsal unpaired median (DUM) neurones. Insect Biochem 9:535–545
Evans P (1978) Octopamine distribution in the insect nervous system. J Neurochem 30:1009–1013
Evans P (1980) Biogenic amines in the insect nervous system. Adv Insect Physiol 15:317–474
Evans P (1985) Octopamine. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiol biochem and pharmacol, vol 11. Pergamon, Oxford, pp 499–531
Evans P, O'Shea M (1978) The identification of an octopaminergic neurone and the modulation of a myogenic rhythm in the locust. J Exp Biol 73:235–260
Ferber M (1989) Are all DUM-neurones octopaminergic? Evidence for peptidergic DUM-neurones in locust abdominal ganglia. In: Erber J, Menzel R, Pflüger HJ, Todt D (eds) Neural mechanism of behavior. Thieme, Stuttgart New York, p 249
Ferber M, Pflüger HJ (1990) Bilaterally projecting neurons in the pregenital abdominal ganglia of the locust: anatomy and peripheral targets. J Comp Neurol 302:447–460
Fifield SM, Finlayson LH (1978) Peripheral neurons and peripheral neurosecretion in the stick insect, Carausius morosus. Proc R Soc Lond (Biol) 200:63–85
Finlayson LH, Osborne MP (1968) Peripheral neurosecretory cells in the stick insect (Carausius morosus) and the blowfly larva (Phormia terrae-novae). J Insect Physiol 14:1793–1801
Geffard M, Buijs RM, Seguela P, Pool CW, LeMoal M (1984) First demonstration of higly specific and sensitive antibodies against dopamine. Brain Res 294:161–165
Goodman CS, Bate M (1981) Neuronal development in the grasshopper. Trends Neurosci 4:163–169
Goodman CS, Pearson KG, Spitzer NC (1980) Electrical excitability: a spectrum of properties in the progeny of a single embryonic neuroblast. Proc Natl Acad Sci USA 77:1676–1680
Goosey MW, Candy DJ (1980) The D-octopamine content of haemolymph of the locust Schistocerca americana gregaria. Insect Biochem 10:393–397
Goosey MW, Candy DJ (1982) The release and removal of octopamine by tissues of the locust Schistocerca americana gregaria. Insect Biochem 12:681–685
Gras H, Hörner M, Runge L, Schürmann FW (1990) Prothoracic DUM neurons of the cricket Gryllus bimaculatus-responses to natural stimuli and activity in walking behavior. J Comp Physiol 166:901–914
Griss C (1989) Serotonin-immunoreactive neurons in the suboesophageal ganglion of the caterpillar of the hawk moth Manduca sexta. Cell Tissue Res 258:101–109
Hahnel C, Bräunig P (1989) Dorsal, unpaired, median (DUM) neurones of the locust suboesophageal ganglion (SOG). In: Elsner N, Singer W (eds) Dynamics and plasticity in neuronal systems. Proceedings 17th Göttingen Neurobiology Conference. Thieme, Stuttgart New York, p 54
Honegger HW, Altman JS, Kien J, Müller-Tautz R, Pollberg E (1984) A comparative study of neck muscle motor neurons in a cricket and a locust. J Comp Neurol 230:517–535
Hoyle G (1974) A function for neurones (DUM) neurosecretory on skeletal muscle of insects. J Exp Zool 189:401–406
Hoyle G (1975) Evidence that insect dorsal unpaired median (DUM) neurons are octopaminergic. J Exp Zool 193:425–431
Hoyle G (1978) The dorsal unpaired median neurons of the locust metathoracic ganglion. J Neurobiol 9:43–57
Hoyle G, Barker DL (1975) Synthesis of octopamine by dorsal median unpaired neurons. J Exp Zool 193:433–439
Johnson B (1966) Fine structure of the lateral cardiac nerves of the cockroach Periplaneta americana (L.). J Insect Physiol 12:645–653
Konings PNM, Vullings HGB, Geffard M, Buijs RM, Diederen JHB, Jansen WF (1988) Immunocytochemical demonstration of octopamine-immunoreactive cells in the nervous system of Locusta migratoria and Schistocerca gregaria. Cell Tissue Res 251:371–379
Kravitz AD (1988) Hormonal control of behavior: amines and the biasing of behavioral output in lobsters. Science 241:1775–1781
Lange AB, Orchard I (1986) Ventral neurons in an abdominal ganglion of the locust, Locusta migratoria, with properties similar to dorsal unpaired median neurons. Can J Zool 64:264–267
Laurent G, Richard D (1986) The organization and role during locomotion of the proximal musculature of the cricket foreleg. I. Anatomy and innervation. J Exp Biol 123:255–283
Livingstone MS, Harris-Warrick RM, Kravitz EA (1980) Serotonin and octopamine produce opposite postures in lobsters. Science 208:76–79
Malamud JG, Mizisin AP, Josephson RK (1988) The effects of octopamine on contraction kinetics and power output of a locust flight muscle. J Comp Physiol [A] 162:827–835
McRae-Degueurce A, Geffard M (1986) One perfusion mixture for immunocytochemical detection of noradrenaline, dopamine, serotonin and acetylcholine in the same rat brain. Brain Res 376:217–219
Miller T, Thomson WW (1968) Ultrastructure of cockroach cardiac innervation. J Insect Physiol 14:1099–1104
Miyamoto T, Shimozawa T (1983) Embryonic development of the central nervous system in the cricket, Gryllus bimaculatus. I. Segmental homologies in early neurogenesis. Zool Mag 92:317–331
Murphy RK (1981) The structure and development of a somatotopic map in crickets: the cercal afferent projection. Dev Biol 88:236–246
Murphy RK (1985) Sprouting and functional regeneration of an identified serotonergic neuron following axotomy. J Neurobiol 16:137–151
Nässel DR, Elekes K (1985) Serotonergic terminals in the neural sheath of the blowfly nervous system: electron microscopical immunocytochemistry and 5.7-dihydroxytryptamine labelling. Neuroscience 15:293–307
O'Gara BA, Drewes CD (1990) Modulation of tension production by octopamine in the metathoracic dorsal longitudinal muscle of the cricket Teleogryllus oceanicus. J Exp Biol 149:161–176
Orchard I, Lange AB (1985) Evidence for octopaminergic modulation of an insect visceral muscle. J Neurobiol 16:171–181
Orchard I, Martin RJ, Sloley BD, Downer RGH (1986) The association of 5-hydroxytryptamine, octopamine, and dopamine with the intrinsic (glandular) lobe of the corpus cardiacum of Locusta migratoria. Can J Zool 64:271–274
Orchard I, Lange AB, Cook H, Ramirez JM (1989) A subpopulation of dorsal unpaired median neurons in the blood-feeding insect Rhodnius prolixus displays serotonin-like immunoreactivity. J Comp Neurol 289:118–128
O'Shea M, Evans PD (1979) Potentiation of neuromuscular transmission by an octopaminergic neurone in the locust. J Exp Biol 79:169–190
Pflüger HJ, Watson AHD (1988) Structure and distribution of dorsal unpaired median (DUM) neurones in the abdominal nerve cord of male and female locusts. J Comp Neurol 268:329–345
Plotnikova SI (1969) Effector neurons with several axons in the ventral nerve cord of Locusta migratoria. J Exp Biochem Physiol 5:339–341
Raabe M (1985) Role of perisympathetic organs and other neurohemal organs in the neurosecretory system of insects. In: Gupta AP (ed) Neurohemal organs of arthropods. Thomas, Springfield Illinois, pp 552–580
Rapus J, Eckert M (1990) A new antiserum to octopamine for the demonstration of neurons with octopamine-like immunoreactivity in the American cockroach. In: Elsner N, Roth G (eds) Brain — perception — cognition. Proceedings 18th Göttingen Neurobiology Conference. Thieme, Stuttgart New York, p 324
Römer H, Marquart V, Hardt M (1988) Organization of a sensory neuropil in the auditory pathway of two groups of Orthoptera. J Comp Neurol 275:201–215
Sombati S, Hoyle G (1984) Generation of behaviors in a locust by local release into neuropil of the natural neuromodulator octopamine. J Neurobiol 15:481–506
Spörhase-Eichmann U, Schürmann FW (1988) Serotonin-immunoreactivity in the central nervous system of the cricket Gryllus bimaculatus. In: Elsner N, Barth FG (eds) Sense organs: interfaces between environment and behavior. Proceedings 16th Göttingen Neurobiology Conference. Thieme, Stuttgart New York, p 298
Spörhase-Eichmann U, Dircksen H, Hecht T, Helle J, Schürmann FW (1991) Neurohaemal-like fibre networks in an insect. In: Elsner N, Penzlin H (eds) Synapse—transmission—modulation. Proceedings 19th Göttingen Neurobiology Conference. Thieme, Stuttgart New York, p 339
Sternberger LA (1979) Immunocytochemistry, 2nd edn. Wiley, New York
Stevenson PA, Pflüger HJ Eckert M, Rapus J (1991) Octopamine immunoreactive neurons in the locust, nerve cord. In: Elsner N, Penzlin H (eds) Synapse — transmission — modulation. Proceedings 19th Göttingen Neurobiology Conference. Thieme, Stuttgart New York, p 1
Taghert PH, Goodman CS (1984), Cell determination and differentiation of identified serotonin-immunoreactive neurons in the grasshopper embryo. J Neurosci 4:989–1000
Tanaka Y, Washio H (1988) Morphological and physiological properties of the dorsal unpaired median neurons of the cockroach metathoracic ganglion. Comp Biochem Physiol 91A:37–41
Thompson KJ, Siegler MVS (1991) Anatomy and physiology of spiking intersegmental interneurons in the median neuroblast lineage of the grasshopper. J Comp Neurol 305:659–675
Tyrer MN, Turner JD, Altman JS (1984) Identifiable neurons in the locust central nervous system that react with antibodies to serotonin. J Comp Neurol 227:313–330
Van der Sluis PJ, Pool CW, Sluiter AA (1988) Press-blotting on gelatin-coated nitrocellulose membranes after gel isoelectric focusing: application in the detection of peptides in the brain and the characterization of antiserum specificity. In: Van Leeuwen FW, Buijs RM, Pool CW, Pach O (eds) Molecular neuroanatomy. Elsevier, Amsterdam, pp 275–288
Watson AHD (1984) The dorsal unpaired neurons of the locust metathoracic ganglion: neuronal structure and diversity, and synapse distribution. J Neurocytol 13:303–327
Wigglesworth VB (1957) The use of osmium in the fixation of tissues. Proc R Soc Lond (Biol) 147:185–199
Wohlers DW, Huber F (1985) Topographical organization of the auditory pathway within the prothoracic ganglion of the cricket Gryllus campestris L. Cell Tissue Res 239:555–565
Woodring JP, Meier OW, Rose R (1988) Effect of development, photoperiod, and stress on octopamine levels in the house cricket, Acheta domesticus. J Insect Physiol 34:759–765
Woodring JP, McBride LA, Fields P (1989) The role of octopamine in handling and exercise-induced hyperglycaemia and hyperlipaemia in Acheta domesticus. J Insect Physiol 35:613–617
Yamaguchi T, Kushiro N, Waki T (1985) Sexual dimorphism of the terminal abdominal ganglion of the cricket. Naturwissen-schaften 72:153–154
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spörhase-Eichmann, U., Vullings, H.G.B., Buijs, R.M. et al. Octopamine-immunoreactive neurons in the central nervous system of the cricket, Gryllus bimaculatus . Cell Tissue Res 268, 287–304 (1992). https://doi.org/10.1007/BF00318798
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318798