Abstract
Projections from peripheral receptors directly into the protocerebrum of insects have only been little studied. Retrograde staining of nerves from the antennae, maxillary palps and legs has revealed some fibres that project into the central areas of the protocerebrum. In the case of the antennae and palps, it was not known which receptors were responsible for these projections. In the legs of locusts, multipolar neurons (MN) with characteristic terminal dendritic masses (TDM) have been described to project into a neuropil called “superior ventral inferior protocerebrum” (SVIP). However, such neurons have only been found in the abdominal infrared organs of the Australian fire beetle Merimna atrata, where they function as thermoreceptors. In several orthopterans, fibres from the antennae and palps also project into the SVIP. The present work suggests that the multipolar neuron from the infrared organ of Merimna also projects into the protocerebrum, possibly into a ventral region functionally analogous to the SVIP. No MNs but single scolopidia were found in the tips of the antennae and palps of locusts, apparently responsible for projections into the SVIP, where they probably function as receptors for haemolymph pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The protocerebrum of the insect brain contains the highest association centres of the insect nervous system such as the mushroom bodies, the lateral horn and the central complex (Strausfeld 2012; Pfeiffer and Homberg 2014; Fahrbach 2006; Gupta and Stopfer 2012). These structures are not present in all the other posterior ganglia. Sensory information processed in the protocerebrum is generally supplied by interneurons or projection neurons and originates from all areas of the insect body. Thus, sensory information provided by ocelli, compound eyes, antennae, and a variety of chemo- and mechanoreceptors located on the mouthparts on the head but also on the thorax or abdomen are fed into the protocerebrum via interneurons downstream of the primary receptor cells (Mizunami 1995; Haag and Borst 2004; Homberg et al. 1989; Bräunig et al. 1981; Yamao et al. 2022). Consequently, interneurons receive inputs from several to very many receptor neurons and perform a first form of information processing such as integration, modulation, and filtering (Kanzaki et al. 1994; Hennig et al. 2004). Finally, in the protocerebrum, multisensory integration is achieved (Kinoshita and Homberg 2017), because the information provided by the different classes of peripheral receptors is combined here.
However, in 8 publications known to the authors so far, direct projections into the protocerebrum were described, which obviously originate from receptor neurons located in the periphery. In 6 cases, these projections were uncovered by anterograde staining of the antennal nerve, where sub-tracts or individual fibres projected into posterior- or ventro-medial regions of the protocerebrum. In particular, such projections were found in larvae of Manduca (Kent and Hildebrand 1987), in honeybees (Maronde 1991; Suzuki 1975; Arnold et al. 1985), in various species of Orthoptera (Ignell et al. 2001), in mosquitoes (Ignell et al. 2005), in cockroaches (Watanabe et al. 2010) and finally also in crickets (Yoritsune and Anomura 2012). In another case, anterograde staining of the maxillary palpal nerve of various orthopterans revealed similar protocerebral projections (Ignell et al. 2000). However, the associated neurons in the antenna and palps have not yet been identified. Therefore, the stimuli measured by these neurons are still unknown.
Finally, in 2013, Bräunig and Krumpholz showed in the migratory locust that such projections are also developed by receptor neurons located in the distal areas of the legs (tibia, tarsus) (Bräunig and Krumpholz 2013). In that work, the associated neurons were described as well. Neuroanatomical and morphological investigations showed that these neurons are multipolar and are equipped with a specialised dendritic areas—named terminal dendritic mass (TDM). A TDM is composed of many hundreds of densely packed dendrites of small diameter. The dendrites contain large amounts of mitochondria. Multipolar neurons with a TDM have been found so far only in the abdominal infrared (IR) organs of pyrophilous beetles of the genus Merimna (includes only the species M. atrata (Schmitz et al. 2000, 2001). The central projections of these neurons working as thermoreceptors in the IR organ of Merimna (Schmitz and Trenner 2003) are unknown.
Although no consistent picture has yet emerged with regard to the exact target sites (neuropiles) of the protocerebral projections mentioned, it seems that, at least in Orthoptera, the projections from the antennae, maxillary palps and legs project into a region that has been termed “superficial ventral inferior protocerebrum” (SVIP). This neuropil is located near the tips of the medial lobes of the mushroom bodies and is characterised by an extreme ventral position (Ignell et al. 2000, 2001; Bräunig and Krumpholz 2013). In the following, we define the protocerebrum as the part of the upper cerebral ganglion immediately adjacent to the brain volume, which contains the antennal lobes (referred to as the deutocerebrum). Thus, we follow the terminology currently in use, based on morphological findings and frequently used in the established literature (Ito et al. 2014; Immonen et al. 2017; Wolff and Strausfeld 2016). However, future developmental genetic studies will have to show whether especially superficially located neuropils of the protocerebrum defined in this way may be of deutocerebral origin from their embryonic origin.
The present work, therefore, has two objectives: (i) identification and morphological characterisation of the receptor neurons (RN) in the antennae and palps of the locust projecting into the SVIP; (ii) elucidation of the central projections of the MN in the IR organ of Merimna. The results will be used to test the hypotheses that MN with TDM also occur in the palps and antennae and that all these neurons project exclusively into the SVIP of the protocerebrum. Most probably, the SVIP is a small part of the crepine that is wrapped around the medial lobes (Ito et al. 2014; Immonen et al. 2017).
Material and methods
Laboratory animals
The Australian fire beetle Merimna atrata (Coleoptera, Buprestidae) and the migratory locust Locusta migratoria (Orthoptera, Acrididae) were studied.
Merimna atrata was captured in January 2019 in Western Australia after forest fires in eucalypt forests on fresh burned areas in the Perth Metropolitan Region (Collection licence number: FO25000232 of the Department of Biodiversity, Conservation and Attractions, WA). The beetles were kept in small containers and fed with raisins, almonds, and peanuts. The neuroanatomical experiments were made in a laboratory at the WA Wildlife Research Centre in Woodvale, WA.
Locusta migratoria was bought in a pet shop (Fressnapf) in Bonn. Until the experiments in the laboratory of the Institute of Zoology at the University of Bonn, the animals were kept in cages and fed with lettuce, carrots, and oatmeal.
The experiments comply with the Principles of Animal Care of the National Institutes of Health, the German Animal Welfare Act and the guidelines of the European Union (Directive 2010/63/EU).
Preparation of the nervous system
To visualise the central projections of the multipolar neurons in the infrared organ of Merimna atrata, all legs of the beetle were removed. Subsequently beetles were glued with their dorsal side to a wooden block using a non-toxic fast-curing 2-component adhesive (Luxatemp from DMG, Hamburg, Germany). In order to keep the running distance of the dye towards the head as short as possible, only nerves running from the anterior pair of IR organs located on the second abdominal segment to the corresponding abdominal ganglion were filled. For this purpose, in the area of the 1st and 2nd sternite, a medial window of approx. 2 mm2 was cut into the cuticle at a distance of about 2 mm from an IR organ. Air sacs and tracheae located in the dissection opening were removed until the nerve running to the IR organ became visible. Finally, the nerve was cut at distance of about 0.5 mm from the ganglion with a dissecting scissors.
In locusts, staining was done on antennal and maxillary palpal nerves in both retrograde and anterograde directions.
To visualise the central projections of the antennal and palpal receptors, antennae and maxillary palps were cut in distal areas. Subsequently the nerves were filled over the terminal openings of the stumps.
In order to selectively fill only peripheral neurons, which run with their central projections into the protocerebrum, a suitable preparative procedure had to be developed. Instead of filling cut nerves with dye, specifically cut parts of the brain were brought into contact with the dye solution in a suitable capillary. Through this approach, only the cut axons of neurons projecting into the protocerebrum were in a dye-exposed area. The antennal lobe remained intact. Consequently, all units already terminating in the antennal lobe were not stained.
For this purpose, a window was frontally cut into the cuticle of the head between the eyes and antennae and the brain was completely exposed (Fig. 1). A metal rod with a flattened tip (width 1 mm) was placed under the ganglion with a micromanipulator. The following sections could now be made on this platform:
-
1.
Retrograde staining of the antennal nerves (cf. Fig. 1A): division of the brain by a median cut close to the antennal lobe. This created the area, in which the cut axons of the neurons projecting into the protocerebrum were located. Subsequently, a cut was made through the optic stalk, and another cut below the tritocerebrum. The brain remnant, therefore, remains connected only to the antennal nerve.
-
2.
Retrograde staining of the maxillary palp nerve (cf. Fig. 1B): the first two sections were made as described above. Then the antennal nerve was cut off and the brain remnant remained connected to the tritocerebrum and the associated connective.
Cutting the brain to size for the anterograde filling of fibres projecting from the protocerebrum into antennae and maxillary palps. Brain (B, i. e. supraoesophageal ganglion) already exposed; frontal view. A For filling the respective neurones in the antenna, 3 cuts were made: 1: A median cut through the brain close to the antennal lobe (AL, black on the right). This cut produced the area finally exposed to the dye. 2: A cut through the thinnest part of the optic lobe. 3: A cut below the tritocerebrum. The residual piece of the brain remains connected only to the antennal nerve. The cut brain was sucked into the dye-filled pipette so that the area created by the first cut became in direct contact to the staining solution. B For filling neurons in the maxillary palps, the first two cuts 1 and 2 described in A were made correspondingly. Cut 4 was made through the antennal nerve. The cut brain was sucked into the pipette as described above
Staining of the receptor neurons and their terminal branches by axonal filling
The ends of the cut nerves (also those in the stumps of the cut antennae and palps) as well as the surfaces of the cut brains containing axons of neurons projecting into the protocerebrum were placed in adapted capillaries with slightly larger diameters. In the case of the brain remnants, a small negative pressure was applied to the lumen of the capillary. The capillaries were filled with a 5% solution of Neurobiotin (in distilled water; Vector Laboratories). Subsequently, the animals with the neural tissue in the capillaries were transferred to humid chambers. The preparations of Merimna atrata, which were filled in anterograde direction, were placed in the refrigerator at a temperature of 8 °C for 5–7 days. Preparations of Locusta migratoria, which were also filled anterogradely, were first stored at room temperature for one day and then placed in the refrigerator for another 2–4 days. Locusta preparations that were filled via the brain remnants into the periphery were first stored at room temperature for 1–3 days and then placed in the refrigerator for further 2–4 days. The vitality of the animals was checked daily, and Ringer solution was replenished if necessary. Subsequently, the parts intended for further processing (antennae, maxillary palps and parts of the central nervous system) were removed and fixed in paraformaldehyde (PFA, 4% in aqua dest.) for 1 to 2 h. If necessary, openings were cut in cuticle to allow a good access for the fixative.
After 5 washing steps (2 × 10 min., 3 × 15 min.) in 0.25% phosphate buffer (PBS), to which 0.1% TritonX was added (PBS-TX), incubation in Cy3™-conjugated streptavidin (1:1000 in PBS-TX, Vector Laboratories, Inc., Burlingame, CA, USA) took place overnight. After further 6 washing steps (3 × 15 min. in PBS-TX, 3 × 15 min. in PBS), the samples were dehydrated using an ascending alcohol series and made transparent by placing them in methyl salicylate (Sigma-Aldrich, Fluka Chemika, Buchs, Switzerland). Finally, the samples were embedded in DPX (Merck KGaA, Darmstadt, HE, Germany) on section slides with a central hollow.
Light and transmission electron microscopy
Freshly excised distal parts of antennae and palps were immediately transferred into iced glutaraldehyde fixative (3% glutaraldehyde in 0.05 mol l−1 cacodylate buffer, pH 7.1, 400 mosmol l−1). Specimens were then fixed overnight, subsequently washed in buffer and postfixed with 1.5% OsO4 in the same buffer. Afterwards specimens were dehydrated through an ascending ethanol series and embedded in Epon 812 using propylene oxide as a bridging solvent. Semithin and ultrathin sections were cut with a Reichert Ultracut Microtome using glass- or diamond knives. Semithin sections with a thickness of 0.5 µm were stained with a 0.05% toluidine-blue/borax solution and examined with a Zeiss Axioscope 5 light microscope. Photos were taken with a Jenoptik Gryphax Arktur. Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a Zeiss Libra 120 TEM at 60 kV.
Fluorescence microscopy
All samples were first examined with an Axioscope 5 (Zeiss, Oberkochen, Germany). Cy3™ fluorescence excitation was performed with the green light LED (565 nm) of a Colibri 3 solid-state light source. Images were taken with an Axiocam 202 mono-digital camera (Zeiss, Oberkochen, Germany).
Selected samples were then further examined with a confocal laser scanning microscope (LSM 710, using a Zeiss AXIO Observer Z1 inverted microscope, Zeiss, Germany) equipped with a tenfold objective. Fluorescence excitation was performed with the green light line of the He/Ne laser at 543 nm. Scanning frequency was set to 400 Hz and resolution in x –y direction was 512 to 512 pixel (pixel size 2.5 µm). Resolution in z-direction was 2 µm. Image stacks of whole ganglia and brains (whole mounts) were acquired with the integrated camera using Zeiss ZEN software, version 3.4.
A two-dimensional representation of the images of a stack, showing all focal planes in one image, was produced with the image processing programme Fiji (ImageJ 1.52n). Three-dimensional reconstruction of the Neurobiotin-filled units was also done using ImageJ software. The reconstructions were then further processed with the ImageJ plugin “SNT Legacy 3D Viewer” and converted into a “surface” representation. Finally, 3D views were created with the Microsoft 3D Viewer.
The illustrations were created with Adobe Photoshop CC (v. 23.2).
Results
The apical scolopidium in the antenna of the locust
The neuroanatomy of antennal neurons that do not remain with their central projections in the antennal lobe but ramify in the protocerebrum were elucidated. For this purpose, the projections of individual fibres in the so-called “superficial ventral inferior protocerebrum” (SVIP) already shown by Bräunig and Krumpholz were confirmed (Fig. 2C).
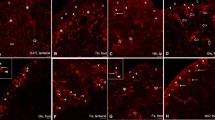
Apical scolopidium in the distal segment of the antenna of Locusta. In A and B: Tips of the antennae on the left. A Light micrograph showing soma (S), scolopale cell (SC) and attachment cell (AC) of the single scolopidium in the terminal segment of the antenna. The attachment cell is in contact to the basal region of the epidermis. The soma region inside the nerve is obviously connected to the terminal opening of the antennal vessel (AV, see arrowhead). N: nerve; Bar: 50 µm. B Soma (arrowhead) and dendritic region of the scolopidium filled with Neurobiotin from the terminal arborisation of its axon in the protocerebrum (indicated by arrow in C). Bar: 50 µm. C Neurobiotin-filled fibres in the brain of Locusta. After filling the antennal nerves of both antennae, the antennal lobes (AL) became visible. From the left AL a fibre is projecting anteriorly into the protocerebrum ramifying in the superior ventral inferior protocerebrum (SVIP, arrow). Although stained much weaker, the projections into the SVIP are also visible on the right. an: anterior, po: posterior; Bar: 200 µm
Selective filling of these units in anterograde direction on the basis of specially cut brains showed that these fibres are the axons of scolopidia (Fig. 2A, B). Due to the long distance and the difficult to control condition that the fibres had to lie in the cut surface of the brain created by the first cut (cf. Fig. 1A), only 2 backfills were successful (in one adult locust and in one last instar larva). However, there were no differences between the two specimens. In each case, only one fibre runs into the terminal antennomere, where it continues into the soma of a scolopidium. From the soma, a long straight dendrite extends further distally (Fig. 2B).
Combined semi/ultrathin section series showed that the soma lies within the nerve (Figs. 2A, 3A). The dendrite inner segment (DIS) separates from the nerve as it continues (Figs. 3B, 4A). Therefore, the dendrite—surrounded by the scolopale and attachment cell—runs freely through the haemocoel (Figs. 4B, 5A) and ends in a cap inside the attachment cell (3F, 5B). Distally, the attachment cell contacts basal areas of the hypodermis (Fig. 2A). The attachment cell is densely filled by microtubuli (Figs. 3E, 4A, B, 5A, B). Six scolopale rods were already found in the apical region of the DIS. The rods are attached to the cell membrane of the DIS by desmosomes and fibres connecting the desmosomes to the cylindrical root filament inside the DIS provide additional rigidity (Fig. 3C). More distal in the region of the dendrite outer segment (DOS), the scolopale rods form an almost closed ring (Fig. 3D); at the level of the ciliary dilatation, individual rods become visible again (Fig. 3E). A striking feature is the nesting of the scolopale and attachment cells. At the level of the DIS, the attachment cell is already recognisable with a basal extension enclosed in a bulge of the scolopale cell (Fig. 4A). Further distal, the attachment cell surrounds the scolopale cell more and more and shows a strong increase in volume (Fig. 4B) until it finally completely surrounds the scolopale cell (Fig. 5A). In the area of the cap, the attachment cell has nearly completely displaced the scolopale cell (Fig. 5B).
Ultrastructure of the scolopidium in the tip of the antenna. A Soma in the periphery of the antennal nerve encased by (dark) glia cells. Asterisk marks large nucleus. Bar: 2 µm. B Cross-section through dendritic inner segment (DIS) which has already separated from the nerve. Arrow points to root filament located in the middle of the DIS. A dark glial cell with a large nucleus (asterisk) surrounds the DIS as well as numerous axons of the antennal nerve. Some axons are marked by arrowheads. Bar: 1 µm. C Cross-section through DIS more distally; DIS surrounded by six scolopale rods. The root filament (asterisk) has adopted a cylindric shape. The rods are enclosed by the membrane of the scolopale cell. In the contact zone between the rods and the DIS desmosomes are located; two are marked by arrowheads. Fine fibres connect the desmosomes to the root filament. The large surrounding scolopale cell shows numerous vacuoles. Bar: 0.5 µm. D Cross-section through the DOS (arrow). Inside the DOS nine double tubuli are visible showing side arms. The scolopale rods have merged for the most part. Bar: 1 µm. E Cross-section through the ciliary dilation (arrow) of the dendritic outer segment. The ciliary dilation shows three additional circular bodies in its centre. From the nine peripheral microtubule doublets, broad bilayered spokes project inward. The scolopale cell containing the scolopale rods is completely surrounded by the attachment cell. A dense arrangement of microtubuli inside the attachment cell causes its dark coloration. Bar: 1 µm. F Cross-section through the cap containing the tip of the DOS (arrowhead). The cap shows a typical fissured structure. The endings of the scolopale rods inside the terminal parts of the scolopale cell are also visible. The complex is fully surrounded by the attachment cell showing a large U-shaped nucleus. Bar: 1 µm. AC: attachment cell, CA: cap, DIS: dendritic inner segment, DOS: dendritic outer segment, GC: glial cell, SC: scolopale cell
Overall view of cross-sections through the scolopidium extending freely through the haemocoel, pt. I. A Section through distal part of the DIS framed by the scolopale rods (corresponding to section shown in Fig. 3C). At this level, the large vacuolated scolopale cell displays a characteristic lacuna containing the basal extension of the attachment cell (arrow). In the lower right a small portion of the nerve accompanied by tracheae and encased by glial cells is still attached to the scolopidium. Bar: 3 µm. B Section through the DOS. The lateral lacuna has become much more extended containing the enlarged attachment cell. Bar: 2.5 µm. AC: attachment cell, DIS: dendritic inner segment, DOS: dendritic outer segment, GC: glial cell, SC: scolopale cell
Overall view of cross-sections through the scolopidium extending freely through the haemocoel, pt. II. A Section through ciliary dilation of the DOS (corresponding to section shown in Fig. 3E). The attachment cell now completely surrounds the scolopale cell. However, last remains of the scolopale cell can still be seen outside this inclusion (asterisk). Bar: 2 µm. B Section through the cap region (corresponding to section shown in Fig. 3F). The large attachment cell encloses the distal part of the scolopale cell and the cap with the DOS in its centre. Note the large nucleus of the attachment cell (asterisk). Bar: 3 µm. AC: attachment cell, CA: cap, DOS: dendritic outer segment, GC: glial cell, SC: scolopale cell
Although no three-dimensional representation of the morphological relationships within the distal antennomere was obtained, there is evidence that the soma region of the scolopidium is in mechanical contact with the terminal opening of the antennal vessel (see arrowhead in Fig. 2A).
As a result, we found a single monodynal, mononematic scolopdium (Field and Matheson 1998).
The apical scolopidium in the maxillary palps of the locust
We also found neurones in the maxillary palps that have terminal arborisations in the SVIP. These projections—already described by Bräunig and Krumpholz in 2013—were confirmed in the context of our work (Fig. 6C). Selective anterograde staining starting at the cut brain surface created by cut 1 (cf. Fig. 1B) revealed one to two somata in the distal areas of the palps (Fig. 6B).
Apical scolopidium in the distal segment of the maxillary palp of Locusta. In A and B tips of the antennae on the left. A Light micrograph showing soma (S), scolopale cell (SC) and attachment cell (AC) of the single scolopidium in the terminal segment of the maxillary palp. The soma is located inside the nerve (N). Arrowhead indicates position where the attachment cell obviously ends. Bar: 50 µm. B Two somata (arrowheads) in the outermost tip of the palpus (left). Units were filled with Neurobiotin from their terminal arborisations in the protocerebrum (indicated by arrow in C). Bar: 100 µm. C Neurobiotin-filled fibres in the brain of Locusta. After filling the nerves of both maxillary palps, neuropil areas of palpal afferents below the antennal lobes in the deutocerebrum became well visible. From both neuropils fibres project anteriorly into the protocerebrum ramifying in the superior ventral inferior protocerebrum (SVIP, marked by arrow on the left). Bar: 200 µm. an: anterior, po: posterior, pr: proximal, di: distal
Subsequent combined series of semi-thin/ultrathin sections revealed that at least the most distal neuron is a scolopidium. Cross-sections through the apical regions of palpi of six animals always showed a scolopidium with a soma located in the nerve (Fig. 6A). As in the antennal scolopidium, the DIS separates from the nerve to run freely in the haemocoel (Figs. 6A, 9A). Longitudinal and cross-sectional series yielded the result in all specimens (N = 6) that the attachment cell terminates freely in the haemocoel. In some cases, thin membranous structures could be detected in the terminal area of the attachment cell (Fig. 6A, arrowhead), but we were unable to identify a structure firmly attached to the attachment cell such as epidermis, other sensory cells, or tracheae. In the proximal area of the DIS the root filament has a star-shaped cross-section (Fig. 7A), which then becomes more and more roundish. Here, the basal parts of the scolopale rods appeared (Fig. 7B). When in the distal area of the DIS, the full number of six scolopale rods is visible, the root filament takes on a pentagonal to hexagonal shape. The reason for this obviously is that fibres arising from the desmosomes that connect the cell membrane to the scolopale rods are centrally attached to the root filament (Fig. 7C). In region of the basal body inside the DIS, the scolopale rods form an almost closed tube (Fig. 7D). This tube becomes completely closed in the region of the DOS (Fig. 7E). Here, the scolopale cell is already completely surrounded by the attachment cell. Further distally, a well-developed ciliary dilatation appears, which in one specimen is supported by processes of the scolopale cell (Figs. 7F, 8A). The attachment cell is surrounded by a relatively thick layer of vortex-shaped extracellular material, which in turn is partially enclosed by a glial cell (Fig. 8A). In the terminal region of the DOS, 9 double tubuli can be seen. Here, also material of the cap becomes visible (Fig. 8B). A few micrometres more distally, the cap is fully developed and encloses the terminal end of the DOS (Fig. 8C). The layer of extracellular material around the attachment cell is still conspicuous. It continues to be partially enclosed by a glial cell. Following the cap, the attachment cell continues surrounded by the vortex-shaped extracellular material for a distance of many micrometres (Fig. 8D) until it finally disappears. Fine lamellar membranous remnants with contacts to the last detectable fragments of the attachment cell suggest that there may be a connection with the basal region of the sensory epithelium covering the apical cap of the palpus.
Ultrastructure of the most distal scolopidium in the tip of the maxillary palp. A Cross-section through dendritic inner segment (DIS) of the scolopidium. Asterisk marks star-shaped root filament located in the middle of DIS. The DIS is enclosed by dark glial cells. Bar: 0.5 µm. B Cross-section through DIS at a more distal position: The root filament (asterisk) has adopted a cylindric shape. The surrounding scolopale cell shows numerous vacuoles. The basal part of a first scolopale rod can be seen on both sides of the duplication of the cell membrane (mesaxon) of the scolopale cell (arrow). Bar: 1 µm. C Section showing the tight connections between the DIS (asterisk) and the scolopale rods. In the contact zone between the rods and the DIS desmosomes are located; two are marked by arrowheads. Fine fibres connect the desmosomes to the root filament inside the DIS. Bar: 0.5 µm. D Cross-section through the lower basal body in the distal part of the DIS. The ciliary root has divided into 9 bundles passing around the periphery of the basal body. On this level, the scolopale rods are close together. Bar: 0.5 µm. E Cross-section through the DOS. Inside the DOS nine microtubule doublets are situated. Each doublet shows an electron-dense A-tubule with a pair of side arms and a hollow B-tubule. The scolopale rods have merged forming a ring of filaments. Bar: 0.25 µm. F Cross-section through the ciliary dilation of the dendritic outer segment. The ciliary dilation shows three additional circular bodies in its centre. From the nine peripheral microtubule doublets, broad bilayered spokes project inward. Bar: 0.25 µm. AC: attachment cell, DIS: dendritic inner segment, DOS: dendritic outer segment, GC: glial cell, SC: scolopale cell
Ultrastructure of the scolopidium in the tip of the maxillary palp. A Another cross-section through a DOS in the area of the ciliary dilation. In this scolopidium, the scolopale cell builds a protoplasmatic bridge supporting the ciliary dilation. The scolopale cell is enveloped by the attachment cell. Both cells are enwrapped by vortex arranged extracellular material. A glial cell partly surrounds this complex. Bar: 1 µm. B Cross-section through the DOS distal to the ciliary dilation. The electron-dense A-tubuli have lost their side arms. Asterisk marks electron-dense material from the emerging cap. Bar: 0.5 µm. C Section through the cap region. The dark attachment cell encloses the distal part of the scolopale cell and the cap with the DOS in its centre (arrowhead). Last distal remains of the scolopale rods are still visible. Bar: 1 µm. D Cross-section through the attachment cell distal from the cap. Note pronounced network of vortex arranged extracellular material around AC. Bar: 0.5 µm. AC: attachment cell, CA: cap, DIS: dendritic inner segment, DOS: dendritic outer segment, GC: glial cell, SC: scolopale cell
In this apical scolopidium also, a strong nesting of scolopale and attachment cell was observed. Compared to the scolopidium located in the antennal tip, the attachment cell is relatively thickly sheathed with extracellular material arranged in a vortex, which in turn is enclosed by glial cells (Fig. 9B). In summary, it is also a monodynal, mononematic scolopdium.
Overall view of cross-sections through the apical scolopidium in the maxillary palp. A Cross-section in which the scolopidium consists mainly of the large scolopale cell. The highly vacuolated scolopale cell encloses the DIS. At this basal position, the scolopale rods are still absent and the ciliary root (asterisk) exhibits a stellate shape. The scolopale cell is surrounded by a thin layer of glial cells. Bar: 3 µm. B Cross-section through DOS (arrow) proximal from the ciliary dilation. The scolopale cell is enclosed by the attachment cell. The scolopale rods form a tube. The complex of the two nested cells is surrounded by vortex-shaped extracellular material in turn partly enclosed by glial cells. Bar: 5 µm. AC: attachment cell, DIS: dendritic inner segment, DOS: dendritic outer segment, GC: glial cell, SC: scolopale cell
Protocerebral projections of units in the nerve running to the IR organ in Merimna atrata
In the nerves connecting the first (anterior) pair of abdominal IR oranges with the 2nd abdominal ganglion, a few units were found that project into the brain (SOG, Fig. 10). Due to the limited number of beetles that all had to be captured in the field and the long distances the Neurobiotin had to travel, only three preparations resulted that showed projections of individual fibres into the SOG. In two specimens, a single fibre is visible in the ipsilateral pharyngeal connective. However, it ends immediately after entering the tritocerebrum. Since no branching is visible, it can be assumed that the transport of dye had stopped at this position.
Protocerebral projection of units in the nerve innervating the IR receptors in Merimna atrata. A Ramification of a single axon in the protocerebrum. The axon is located in the nerve running from the IR organ to the associated abdominal ganglion. Bar: 150 µm. B Schematic drawing of the protocerebral projection showing the antennal lobes and the upper and lower central bodies as lead structures. Bar: 150 µm. C Dorsal view of the ramification area at higher magnification. Upper and lower central bodies are visible as well as the noduli (arrows). One collateral runs further anteriorly (arrowhead). Bar: 100 µm. D Confocal image of a lateral view of the brain of Merimna showing the ramification area of the axon located ventral from the central bodies. Position of the central body marked with dashed lines. Arrowhead points to one collateral running further anteriorly. Bar: 100 µm. AL: antennal lobe, CB: central body, CBU, CBL: upper and lower central body, CC: circumesophageal connective, an: anterior, po: posterior, pr: proximal, di: distal, la: lateral, ce: central
In another specimen, a fibre could be traced into the inferior neuropils of the brain (Ito et al. 2014) (Fig. 10A–D). Related to body axis orientation, the terminal arborisations obviously take place in a neuropil region located anterior to the central body of the mushroom body (Fig. 10C, D). If it should turn out that it is also anterior to the medial lobe, arborisations may be within the crepine as well. Similar to the arborisations shown in the brain of Locusta in the SVIP, a fibre collateral also runs from this branching region further anteriorly (arrowheads in Figs. 10C, D).
Discussion
Projections of primary afferents into the protocerebrum
As mentioned in the introduction, direct projections of sensory neurons into the protocerebrum have not been well described. In most cases, these are incidental findings that have arisen in the course of investigations into other questions. As a result, there is no consistent picture with regard to the number of fibres (one to several dozen, i.e. fibre tracts), projection areas (neuropils) and, in particular, the modality of the associated sensory cells. Since almost exclusively retrograde staining of antennal nerves into the CNS has been performed, no information about the somata located in the periphery has been obtained, with one exception (multipolar neurons with a presumed thermosensitive function in the legs of Locusta, see below).
In bees, crickets and cockroaches, tracts have been described that branch from the antennal nerves and then run in the median regions of the PC. In the bee, tracts extend into the median but also into the lateral posterior PC. In these regions, the fibres appear to overlap with fibres from visual projection neurons that also branch there (Maronde 1991). In crickets and cockroaches, fibre tracts run from the antennal nerve into medioventral regions of the PC (Watanabe et al. 2010; Yoritsune and Aonuma 2012).
One to very few (2–3) fibres of the antennal nerve have been found in Manduca caterpillars and mosquitos, which also run into medioventral regions of the PC. In Manduca caterpillars, 1–2 fibres initially extend into the median PC, run dorsally and eventually branch out into uncharacterised regions of the brain (Kent and Hildebrand 1987). In mosquitoes, a few ventrally arranged fibres enter both the superior and inferior median PC (Ignell et al. 2005).
Finally, in several Orthoptera species, retrograde staining of the nerves of the antennae, maxillary palps and legs identified a few fibres each that all enter a ventral neuropil of the PC. Because of its ventral location, this neuropil was named the “superficial ventral inferior protocerebral” neuropil (SVIP) (Ignell et al. 2000, 2001; Bräunig and Krumpholz 2013). It lies in close proximity to the tips of the ß-lobes of the mushroom bodies. However, some fibres extend beyond the SVIP further anteriorly into as yet undefined protocerebral regions. These fibres are particularly prominent when the antennal nerve is stained. In the case of the leg nerves, anterograde staining and subsequent histological and ultrastructural studies revealed that a few multipolar neurons located in the legs, which have a so-called “terminal dendrite mass” (TDM), are responsible for the projections into the SVIP. A TDM is characterised by the striking feature that the dendrites branch out strongly and lose a lot of diameter at the same time. As the dendrites stay together, a compact, tangle-like dendritic mass is formed, characterised by large amounts of mitochondria inside the dendrites. One of these neurons was found in the proximal tibia and two in the tarsi. The somata are located in the nerves or directly in the area of nerve branches (Bräunig and Krumpholz 2013). Before their discovery in the legs, MNs with a TDM had only been described in the abdominal infrared organs of the Australian ‘fire beetle’ Merimna atrata. Here, one MN innervates each IR organ and functions as a thermoreceptor (Schmitz et al. 2000, 2001; Schmitz and Trenner 2003).
Protocerebral projections from the IR organs of Merimna atrata
The hypothesis described in the Introduction that the MN with a TDM innervating the IR organs of Merimna also projects into the protocerebrum could be confirmed with high probability by the results of this work. However, a neuropil region morphologically identical and, therefore, possibly homologous to the SVIP neuropil in the locust brain could not be shown by our neuroanatomical staining. Due to the different structure of the orthopteran and beetle brains, it is possible that a SVIP neuropil does not exist in beetles. On the other hand, the fibre shown in Fig. 10 has a similar median course like the fibres that branch out in the orthopteran SVIP. Although the presumed areas where synaptic contacts with interneurons are formed are not as clearly concentrated and are not located as far ventral as in the orthopteran SVIP, parts of the fibres also extend further anteriorly in the Merimna protocerebrum (arrowhead in Fig. 10C). It can, therefore, be postulated that the protocerebral region stained here may be functionally analogous to the SVIP of orthopterans because they may make contacts with spatially similarly situated interneurons. Further neuroanatomical studies of the antennal and maxillary palpal nerves will have to show whether there are also neurons in the antennal and maxillary palps of Merimna that ramify in the protocerebrum in a similar way as the fibres of the nerve that run to the IR organ.
Until the MN is filled intracellularly with dye, it cannot be excluded that afferents of other receptors are responsible for the projections found in the protocerebrum. The nerve into which the MN axon enters continues laterally beyond the IR organ. Various mechanoreceptors are known to be located in this area: exteroreceptors such as cuticular hair mechanoreceptors (sensilla trichodea) and interoreceptors such as chordotonal organs and multipolar stretch receptors. A small chordotonal organ (CO) of unknown function consisting of 2 scolopidia is also located immediately adjacent to the MN innervating the IR organ. However, protocerebral projections of these mechanoreceptors have not been described. The most anterior projections of abdominal mechanoreceptors known so far originate from COs housed in the abdominal tymbal organs of stinkbugs projecting to the AMMC of the deutocerebrum (Nishino et al. 2016).
It is, therefore, postulated that the protocerebral projections of the fibres shown here, which lie in the nerve running to the IR organ, originate from the thermoreceptive MN in the IR organ. Like in the SVIP of the locust brain, the connections of the axonal terminals in the protocerebrum to secondary interneurons have to be elucidated. The focus of interest is primarily on connections to the visual systems.
Protocerebral projections of single scolopidia in antennae and palps
Since retrograde staining of the antennal and maxillary palpal nerves of the Orthoptera species studied revealed projections of some fibres into the SVIP (Ignell et al. 2000, 2001; Bräunig and Krumpholz 2013), and later on Bräunig and Krumpholz were able to show that MN with TDM in the legs of Locusta also project into the SVIP, the authors hypothesised that MN with TDM must also be present in the antennal and maxillary palps of Orthoptera. Therefore, an important aim of the present study was to demonstrate these MN by anterograde staining and morphological examination in the two head appendages.
Surprisingly, only single scolopidia was found in the apical region of the antennae and maxillary palps, obviously projecting with their axons into the SVIP. As scolopidia are generally present on all parts of the insect body (Field and Matheson 1998; Moulins 1976), scolopidia have already been described also in the tips of the antennae and palps. A single scolopidium has been found in the terminal segment of the antenna of the last instar larva of Tenebrio molitor. As in the locust antenna, the attachment cell is connected to the basal membrane of the hypodermis (Bloom et al. 1981). In the labial palps of pupae of several butterfly species, 3 scolopidia each have been found in very distal positions (Lee and Altner 1986; Lee et al. 1988). However, in all cases, the somata were not located in a nerve. In contrast, the somata of the scolopidia found in our study in the locust antenna were located in the nerves. Since the somata in the antennae are located in the distal antennomere and in the apical 3rd segment of the palps, a function as a stretch receptor measuring distance changes between 2 articulated sclerotised segments can be excluded. In the antenna, the region of the soma is obviously connected to the terminal opening the antennal vessel; in the palps, the attachment cell is most probably connected to the basal area of the hypodermis, in which the sensory and enveloping cells of the sensilla are embedded. These external sensilla are located in large numbers on the membranous dome-shaped apex of the palpus. This region of soft cuticle can be inflated and deflated by haemolymph pressure. Therefore, we speculate that the scolopidium may measure the position of the membranous apical region of the palp. In the case of the antenna, the rhythmic activity of the basal antennal heart (Pass 1980) alters the diameter of the apical orifice of the antennal vessel. These events could be measured by the scolopidium, which in this case would be involved in measuring haemolymph pressure and thus may be involved in circulation regulation. The authors of the three former studies mentioned above also speculated that the function of the scolopidia, termed “apical sensory organs” (ASO), might be to sense changes in cuticular tension and thus measure the hydraulic pressure of the haemolymph. A comprehensive description of how blood pressure is coupled with cuticle deformation in the insect antenna has been published recently (Donley et al. 2022). However, nothing seems to be known about internal mechanosensors involved in blood pressure regulation.
Concluding remarks
The hypothesis of Bräunig and Krumpholz that MNs with TDM with projections into the SVIP may be present in all body appendages—at least in Orthoptera—could not be confirmed by the present work. The fact that scolopidia were found in the distal areas of the antennae and maxillary palps, which are obviously responsible for these projections, makes the issue of protocerebral projections from peripheral receptors even more confusing. However, assuming that a thermoreceptive function of the MNs in the legs can be demonstrated, additional thermoreceptors in the antenna would make comparatively little sense, since the insect antenna is equipped with well-studied thermoreceptors (Altner and Loftus 1985).
In the future, it would, therefore, be necessary to investigate which types of interneurons have synaptic contacts with the receptors that branch out in the SVIP and to which other brain areas these interneurons in turn pass on information. Physiological characterisation of these interneurons would require intracellular recordings and staining.
Availability of data and materials
Acquired data are presented in this study, raw images can be made available upon personal request to the authors.
Code availability
Not applicable.
References
Altner H, Loftus R (1985) Ultrastructure and function of insect thermo- and hygroreceptors. Ann Rev Entomol 30:273–295
Arnold G, Masson C, Budharugsa S (1985) Comparative study of the antennal lobes and their in the worker bee and the drone (Apis mellifera). Cell Tissue Res 242:593–605
Bloom J, Zacharuk R, Holodniuk A (1981) Ultrastructure of a terminal chordotonal sensillum in larval antennae of the yellow mealworm, Tenebrio molitor L. Can J Zool 59(3):515–524
Bräunig P, Krumpholz K (2013) Internal receptors in insect appendages project directly into a special brain neuropile. Front Zool 10(1):54
Bräunig P, Hustert R, Pflüger HJ (1981) Distribution and specific central projections of mechanoreceptors in the thorax and proximal leg joints of locusts: I: morphology, location and innervation of internal proprioceptors of pro- and metathorax and their central projections. Cell Tissue ReS. https://doi.org/10.1007/bf00234545
Donley G, Sun Y, Pass G, Adler PH, Beard CE, Owens J, Kornev KG (2022) Insect antennae: coupling blood pressure with cuticle deformation to control movement. Acta Biomater 147:102–119
Fahrbach SE (2006) Structure of the mushroom bodies of the insect brain. Annu Rev Entomol 51:209–232
Field LH, Matheson T (1998) Chordotonal organs of insects. Adv Insect Physiol 27:1–228
Gupta N, Stopfer M (2012) Functional analysis of a higher olfactory center, the lateral horn. J Neurosci 32(24):8138–8148
Haag J, Borst A (2004) Neural mechanism underlying complex receptive field properties of motion-sensitive interneurons. Nat Neurosci 7(6):628–634
Hennig RM, Franz A, Stumpner A (2004) Processing of auditory information in insects. Microsc Res Tech 63(6):351–374. https://doi.org/10.1002/jemt.20052
Homberg U, Christensen TA, Hildebrand JG (1989) Structure and function of the deutocerebrum in insects. Ann Rev Entomol 34:477–501
Ignell R, Anton S, Hansson BS (2000) The maxillary palp sensory pathway of Orthoptera. Arthropod Struct Dev 29(4):295–305
Ignell R, Anton S, Hansson BS (2001) The antennal lobe of orthoptera—anatomy and evolution. Brain Behav Evol. https://doi.org/10.1159/000047222
Ignell R, Dekker T, Ghaninia M, Hansson BS (2005) Neuronal architecture of the mosquito deutocerebrum. J Comp Neurol 493(2):207–240
Immonen EV, Dacke M, Heinze S, El Jundi B (2017) Anatomical organization of the brain of a diurnal and a nocturnal dung beetle. J Comp Neurol 525(8):1879–1908
Ito K, Shinomiya K, Ito M, Armstrong JD, Boyan G, Hartenstein V, Harzsch S, Heisenberg M, Homberg U, Jenett A (2014) A systematic nomenclature for the insect brain. Neuron 81(4):755–765
Kanzaki R, Ikeda A, Shibuya T (1994) Morphological and physiological properties of pheromone-triggered flipflopping descending interneurons of the male silkmoth Bombyx mori. J Comp Physiol A 175:1–14
Kent KS, Hildebrand JG (1987) Cephalic sensory pathways in the central nervous system of larval Manduca sexta (Lepidoptra: Sphingidae). Philos Trans Roy Soc London. https://doi.org/10.1098/rstb.1987.0001
Kinoshita M, Homberg U (2017) Insect brains: minute structures controlling complex behaviors. Brain evolution by design. Springer, Tokyo, pp 123–151
Lee J-K, Altner H (1986) Structure, development and death of sensory cells and neurons in the pupal labial palp of the butterflies Pieris rapae L. and Pieris brassicae L. (Insecta, Lepidoptera). Cell Tissue Res 244:371–383
Lee J-K, Kim C-W, Altner H (1988) Differences in degeneration of the apical scolopidial organ in the labial palp of Lepidoptera during pupal development. Zoomorphology 108(2):77–83
Maronde U (1991) Common projection areas of antennal and visual pathways in the honeybee brain, Apis mellifera. J Comp Neurol 309(3):328–340
Mizunami M (1995) Functional diversity of neural organization in insect ocellar systems. Vision Res 35:443–452
Moulins M (1976) Ultrastructure of chordotonal organs. In: Mill PJ (ed) Structure and function of proprioceptors in the invertebrates. Chapman and Hall Ltd, London, pp 387–425
Nishino H, Mukai H, Takanashi T (2016) Chordotonal organs in hemipteran insects: unique peripheral structures but conserved central organization revealed by comparative neuroanatomy. Cell Tissue Res 366(3):549–572
Pass G (1980) The anatomy and ultrastructure of the antennal circulatory organs in the cockchafer beetle Melolontha melolontha L. (Coleoptera, Scarabaeidae). Zoomorphology 96(1–2):77–89
Pfeiffer K, Homberg U (2014) Organization and functional roles of the central complex in the insect brain. Annu Rev Entomol 59:165–184
Schmitz H, Trenner S (2003) Electrophysiological characterization of the multipolar thermoreceptors in the “fire-beetle” Merimna atrata and comparison with the infared sensilla of Melanophila acuminata (both Coleoptera, Buprestidae). J Comp Physiol A 189:715–722
Schmitz H, Schmitz A, Bleckmann H (2000) A new type of infrared organ in the Australian “fire-beetle” Merimna atrata (Coleoptera, Buprestidae). Naturwissenschaften 87:542–545
Schmitz H, Schmitz A, Bleckmann H (2001) Morphology of a thermosensitive multipolar neuron in the infrared organ of Merimna atrata (Coleoptera, Buprestidae). Arthropod Struct Dev. https://doi.org/10.1016/s1467-8039(01)00028-7
Strausfeld NJ (2012) Arthropod brains: evolution, functional elegance, and historical significance. Belknap Press, Cambridge
Suzuki H (1975) Antennal movements induced by odour and central projection of the antennal neurones in the honey-bee. J Insect Physiol 21(4):831–847
Watanabe H, Nishino H, Nishikawa M, Mizunami M, Yokohari F (2010) Complete mapping of glomeruli based on sensory nerve branching pattern in the primary olfactory center of the cockroach Periplaneta americana. J Comp Neurol 518(19):3907–3930
Wolff G, Strausfeld NJ (2016) The insect brain: a commentated primer. In: Schmidt-Rhaesa A, Purschke G (eds) Structure and Evolution of Invertebrate Nervous Systems. Oxford University Press, Oxford, pp 597–639
Yamao H, Shidara H, Ogawa H (2022) Central projections of cercal giant interneurons in the adult field cricket, Gryllus bimaculatus. J Comp Neurol 530:2372–2384
Yoritsune A, Aonuma H (2012) The anatomical pathways for antennal sensory information in the central nervous system of the cricket, Gryllus bimaculatus. Invert Neurosci 12(2):103–117
Acknowledgements
We are indebted to the Department of Biodiversity, Conservation and Attractions (DBCA) in WA for issuing the Fauna Taking Licences No. FO25000232 from 06/01/2020. Brian Inglis, Clayton Sanders and Leigh Sage from the Parks and Wildlife Service of DBCA in Wanneroo permitted access to the burnt areas and supported our fieldwork. John Angus from the DBCA Science Division provided lab space in the Woodvale Research Centre in Woodvale, WA.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study has been supported by grant SCHM 1161/16-1 from the German Research Foundation (DFG) to HS.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Neuroanatomical experiments were performed by M. Hinz, cutting and processing of ultrathin sections was made by A. Schmitz, and TEM micrographs were taken by A. Schmitz and H. Schmitz. The first draft of the manuscript was written by H. Schmitz, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Ethical approval
No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with an unregulated invertebrate species.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hinz, M., Schmitz, A. & Schmitz, H. Neuroanatomy and functional morphology of peripheral receptor neurones with direct projections into the protocerebrum of the brains of the locust and a jewel beetle. Zoomorphology 142, 341–356 (2023). https://doi.org/10.1007/s00435-023-00606-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-023-00606-7