Abstract
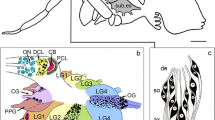
We have used a cytochemical technique to investigate the distribution of acetylcholinesterase (AChE) activity in the deutocerebrum of the brain of the sphinx moth Manduca sexta. To distinguish between extra-and intracellular pools of the enzyme, some brains were treated prior to histochemical staining with echothiophate, an irreversible AChE inhibitor which penetrates cell membranes very slowly and, therefore, inhibits only extracellular AChE. In the antennal nerve, fascicles of presumably mechanosensory fibers show echothiophateinsensitive AChE activity. They bypass the antennal lobe and project to the antennal mechanosensory and motor center of the deutocerebrum. In the antennal lobe, fibers in the coarse neuropil, cell bodies in the lateral cell group, and all glomeruli exhibit AChE activity. In most ordinary glomeruli, echothiophate-sensitive AChE activity is concentrated in the outer cap regions, corresponding to the terminal arborizations of olfactory afferents. A previously unrecognized glomerulus in the ventro-median antennal lobe shows uniform and more intense AChE-specific staining that the other glomeruli. No AChE activity appeared to be associated with malespecific pheromone-sensitive afferents in the macro-glomerular complex. About 67 interneurons with somata in the lateral cell group of the antennal lobe show echo-thiophate-insensitive AChE activity. These neurous seem to be members of two types of antennal-lobe projection neurons with fibers passing through the outer-antenno-cerebral tract to the protocerebrum. AChE-stained arborizations of these neurons appear to invade all glomeruli, including three distinguishable subunits of the male-specific macroglomerular complex. In echothiophate-treated animals, the projections of one of these types of fiber form large terminals in the lateral horn of protocerebrum, which partly protrude into the adjacent glial cell layer. The results suggest that extracellularly accessible AChE is associated with ordinary olfactory receptor terminals but apparently not with pheromone-sensitive afferents. Intracellular AChE appears to be present in antennal mechanosensory fibers and in two types of olfactory projection neurons of the antennal lobe. The study provides further evidence for cholinergic neurotransmission of most antennal afferents. The AChE-containing interneurons might be cholinergic as well or use the enzyme for functions unrelated to hydrolysis of acetylcholine.
Similar content being viewed by others
Abbreviations
- ACh :
-
acetylcholine
- AChE :
-
acetylcholinesterase
- AL :
-
antennal lobe
- AMMC :
-
antennal mechanosensory and motor center
- ChAT :
-
choline acetyltransferase
- IACT :
-
inner antenno-cerebral tract
- MGC :
-
macroglomerular complex
References
Appleyard ME (1992) Secreted acetylcholinesterase: non-classical aspects of a classical enzyme. Trends Neurosci 15:485–490
Bell RA, Joachim FA (1976) Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann Entomol Soc Am 69:365–373
Brimijoin S, Skau K, Wiermaa MJ (1978) On the origin and fate of external acetylcholinesterase in peripheral nerve. J Physiol 285:143–158
Buchner E, Buchner S, Crawford G, Mason WT, Salvaterra PM, Sattelle DB (1986) Choline acetyltransferase-like immunoreactivity in the brain of Drosophila melanogaster. Cell Tissue Res 246:57–62
Camazine SM, Hildebrand JG (1979) Central projections of antennal sensory neurons in mature and developing Manduca sexta. Soc Neurosci Abstr 5:492
Christensen TA, Hildebrand JG (1987) Functions, organization, and physiology of the olfactory pathways in the lepidopteran brain. In: Gupta AP (ed) Arthropod brain: its evolution, development, structure and functions. Wiley, New York, pp 457–484
Duve H, Thorpe A (1989) Distribution and functional significance of Met-enkephalin-Arg6-Phe7 and Met-enkephalin-Arg6-Gly7-Leu8-like peptides in the blowfly Calliphora vomitoria. I. Immunocytochemical mapping of neuronal pathways in the brain. Cell Tissue Res 258:147–161
Emson PC, Burrows M, Fonnum F (1974) Levels of glutamate decarboxylase, cholineacetyltransferase and acetylcholinesterase in identified motoneurons of the locust. J Neurobiol 5:33–42
Fournier D, Mutero A, Pralavorio M, Bride JM (1992) Drosophila acetylcholinesterase: analysis of structure and sensitivity to in secticides by in vitro mutagenesis and expression. In: Shafferman A, Velan B (eds) Multidisciplinary approaches to cholinesterase functions. Plenum Press, New York, pp 75–81
Frontali N, Piazza R, Scopelliti R (1971) Localization of acetylcholinesterase in the brain of Periplaneta americana. J Insect Physiol 17:1833–1842
Gorczyca MG, Hall JC (1987) Immunohistochemical localization of choline acetyltransferase during development and in Cha ts mutants of Drosophila melanogaster. J Neurosci 7:1361–1369
Greenfield S (1984) Acetylcholinesterase may have novel functions in the brain. Trends Neurosci 7:364–368
Greenfield SA (1992) Acetylcholinesterase as a modulatory neuroprotein and its influence on motor control. In: Shafferman A, Velan B (eds) Multidisciplinary approaches to cholinesterase functions. Plenum Press, New York, pp 233–242
Hansson BS, Christensen TA, Hildebrand JG (1991) Functionally distinct subdivisions of the macroglomerular complex in the antennal lobe of the male sphinx moth Manduca sexta. J Comp Neurol 312:264–278
Hawkins CA, Greenfield SA (1992a) Non-cholinergic action of exogenous acetylcholinesterase in the rat substantia nigra I: Differential effects on motor behavior. Behav Brain Res 48:153–157
Hawkins CA, Greenfield SA (1992b) Non-cholinergic action of exogenous acetylcholinesterase in the rat substantia nigra II: Long-term interactions with dopamine metabolism. Behav Brain Res 48:159–163
Hildebrand JG (1980) Development of putative acetylcholine receptors in normal and deafferented antennal lobes during metamorphosis of Manduca sexta. In: Sattelle DB, Hall LM, Hildebrand JG (eds) Receptors for neurotransmitters, hormones and pheromones in insects. Elsevier/North-Holland, Amsterdam, pp 209–220
Hildebrand JG (1985) Metamorphosis of the insect nervous system. Influences of the periphery on the postembryonic development of the antennal sensory pathway in the brain of Manduca sexta. In: Selverston AI (ed) Model neural networks and behavior. Plenum Press, New York, pp 129–148
Hildebrand JG, Hall LM, Osmond BC (1979) Distribution of binding sites for 125I-labeled α-bungarotoxin in normal and deafferented antennal lobes of Manduca sexta. Proc Natl Acad Sci USA 76:499–503
Hildebrand JG, Matsumoto SG, Camazine SM, Tolbert LP, Blank S, Ferguson H, Ecker V (1980) Organization and physiology of antennal centres in the brain of the moth Manduca sexta. In: Insect Neurobiology and pesticide action (Neurotox '79). Society of Chemical Industry, London, pp 375–382
Homberg U (1990) Immunocytochemical demonstration of transmitter candidates in the central olfactory pathways in the sphinx moth Manduca sexta. In: Døving KB (ed) Olfaction and Taste X, GSC A/S, Oslo, pp 151–158
Homberg U (1994) Distribution of neurotransmitters in the insect brain. Progress in Zoology, vol 40. Fischer, Stuttgart Jena New York
Homberg U, Montague RA, Hildebrand JG (1988) Anatomy of antenno-cerebral pathways in the brain of the sphinx moth Manduca sexta. Cell Tissue Res 254:255–281
Homberg U, Christensen TA, Hildebrand JG (1989) Structure and function of the deutocerebrum in insects. Annu Rev Entomol 34:477–501
Karnovsky MJ, Roots L (1964) A “direct-coloring” thiocholine method for cholinesterase. J Histochem Cytochem 12:219–221
Kent KS, Harrow ID, Quartararo P, Hildebrand JG (1986) An accessory olfactory pathway in Lepidoptera: the labial pit organ and its central projections in Manduca sexta and certain other sphinx moths and silk moths. Cell Tissue Res 245:237–245
Kreissl S, Bicker G (1989) Histochemistry of acetylcholinesterase and immunocytochemistry of an acetylcholine receptor-like antigen in the brain of the honeybee. J Comp Neurol 286:71–84
Lester DS, Gilbert LI (1987) Characterization of acetylcholin-esterase activity in the larval brain of Manduca sexta. Insect Biochem 17:99–109
Masson C, Strambi C (1977) Sensory antennal organization in an ant and a wasp. J Neurobiol 8:537–548
Massoulié J, Bon S,(1982) The molecular forms of cholinesterases in vertebrates. Annu Rev Neurosci 5:57–106
Massoulié J, Bon S, Kreijci E, Coussen F, Duval N, Chatel J-M, Anselmet A, Legay C, Vallette F-M (1991) The structure and significance of multiple cholinesterase forms. In: Massoulié J, Bacou F, Barnard E, Chatonnet A, Doctor BP, Quinn DM (eds) Cholinesterases. Structure, function, mechanism, genetics, and cell biology. American Chemical Society, Washington, pp 2–6
Massoulié J, Pezzementi L, Bon S, Kreijci E, Vallettes F-M (1993) Molecular and cellular biology of cholinesterases. Progr Neurobiol 41:31–91
Maxwell GD, Tait JF, Hildebrand JG (1978) Regional synthesis of neurotransmitter candidates in the CNS of the moth Manduca sexta. Comp Biochem Physiol [C] 61:109–119
Mutero A, Fournier D (1991) Drosophila acetylcholinesterase structure. In: Massoulié J, Bacou F, Barnard E, Chatonnet A, Doctor BP, Quinn DM (eds) Cholinesterases Structure, function, mechanism, genetics, and cell biology. American Chemical Society, Washington, pp 141–145
Prescott DJ, Hildebrand JG, Sanes JR, Jewett S (1977) Biochemical and developmental studies of acetylcholine metabolism in the central nervous system of the moth Manduca sexta. Comp Biochem Physiol [C] 56:77–84
Rospars JP, Hildebrand JG (1992) Anatomical identification of glomeruli in the antennal lobes of the male sphinx moth Manduca sexta. Cell Tissue Res 270:205–227
Sanes JR, Hildebrand JG (1976a) Structure and development of antennae in a moth, Manduca sexta. Dev Biol 51:282–299
Sanes JR, Hildebrand JG (1976b) Acetylcholine and its metabolic enzymes in developing antennae of the moth, Manduca sexta. Dev Biol 52:105–120
Sanes JR, Prescott DJ, Hildebrand JG (1977) Cholinergic neurochemical development of normal and deafferented antennal lobes in the brain of the moth, Manduca sexta, during metamorphosis. Brain Res 119:389–402
Schuster R, Phannavong B, Schröder C, Gundelfinger ED (1993) Immunohistochemical localization of a ligand-binding and a structural subunit of nicotinic acetylcholine receptors in the central nervous system of Drosophila melanogaster. J Comp Neurol 335:149–162
Small DH (1991) Acetylcholinesterases: proteases regulating cell growth and development? In: Massoulié J, Bacou F, Barnard EA, Chattonnet A, Doctor BP, Quinn DM (eds) Cholinesterases. Structure, function, mechanism, genetics, and cell biology, American Chemical Society, Washington, pp 374–378
Stengl M, Homberg U, Hildebrand JG (1990) Acetylcholinesterase activity in antennal receptor neurons of the sphinx moth Manduca sexta. Cell Tissue Res 262:245–252
Tago H, Kimura H, Maeda T (1986) Visulization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J Histochem Cytochem 34:1431–1438
Taylor P, Schumacher M, MacPhee-Quigley K, Friedmann T, Taylor S (1987) The structure of acetylcholinesterase: relationship to its function and cellular disposition. Trends Neurosci 10:93–95
Tolbert LP, Matsumoto SG, Hildebrand JG (1983) Development of synapses in the antennal lobes of the moth Manduca sexta during metamorphosis. J Neurosci 3:1158–1175
Toutant J-P (1989) Insect acetylcholinesterase: catalytic properties, tissue distribution and molecular forms. Progr Neurobiol 32:423–446
Trimmer BA, Weeks JC (1989) Effects of nicotimic and muscarinic agents on an indentified motoneurone and its direct afferent inputs in larval Manduca sexta. J Exp Biol 144:303–337
Veenstra JA, Romberg-Privee HM, Schooneveld H, Polak JM (1985) Immunocytochemical localization of peptidergic neurons and neurosecretory cells in the neuroendocrine system of the Colorado potato beetle with antisera to vertebrate regulatory peptides. Histochemistry 82:9–18
Waldrop B, Hildebrand JG (1989) Physiology and pharmacology of acetylcholinergic responses of interneurons in the antennal lobes of the moth Manduca sexta. J Comp Physiol [A] 164:433–441
Wallace B, Gillon JW (1982) Characterization of acetylcholin-esterase in individual neurons in the leech central nervous system. J Neurosci 2:1106–1118
Weeks JC, Jacobs GA (1987) A reflex behavior mediated by monosynaptic connections between hair afferents and motoneurons in the larval tobacco hornworm, Manduca sexta. J Comp Physiol [A] 160:315–329
Wolfgang WJ, Forte WA (1989) Expression of acetylcholinesterase during visual system development in Drosophila. Dev Biol 131:321–330
Zimmermann B (1992) Untersuchungen zur Morphogenese, sensorischen Transduktion and zentralen Projektion antennaler Sensillen des Seidenspinners Antheraea pernyi. Ph.D. thesis, University of Regensburg, Germany
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Homberg, U., Hoskins, S.G. & Hildebrand, J.G. Distribution of acetylcholinesterase activity in the deutocerebrum of the sphinx moth Manduca sexta. Cell Tissue Res 279, 249–259 (1995). https://doi.org/10.1007/BF00318481
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318481