Summary
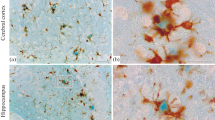
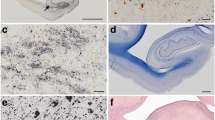
In this immunocytochemical study, the brains of nine squirrel monkeys (Saimiri sciureus), ranging from 8 to 27 years of age, were examined for the presence and distribution of β/A4 amyloid, a 4-kilodalton peptide. In aged squirrel monkeys, amyloid is associated primarily with intracerebral and meningeal capillaries and arterioles and occurs to a lesser degree as small and/or diffuse deposits in the neural parenchyma and in the dense cores of senile plaques. Cerebrovascular amyloid is found primarily in neocortex, amygdala, and septum verum and is rare or nonexistent in other subcortical gray structures, white matter, cerebellum, and spinal cord; this pattern of localization is comparable to that in humans with cerebral amyloid angiopathy. There is a significant correlation between cerebrovascular and parenchymal deposits of amyloid. However, cerebrovascular amyloid is always the most abundant form in squirrel monkeys, even in cases of severe cerebral amyloidosis. In contrast to squirrel monkeys, aged rhesus monkeys (Macaca mulatta) develop, mostly parenchymal deposits of amyloid and have relatively less vascular amyloid. This species difference in the histological distribution of amyloid suggests that separate mechanisms may influence the accumulation of amyloid in cerebral blood vessels and in the neural parenchyma. These data also indicate that the squirrel monkey can serve as a model for investigations of cerebrovascular amyloidosis.
Similar content being viewed by others
References
Allsop D, Landon M, Kidd M, Lowe JS, Reynolds GP, Gardner A (1986) Monoclonal antibodies raised against a subsequence of senile plaque core protein react with plaque cores, plaque periphery and cerebrovascular amyloid in Alzheimer's disease. Neurosci Lett 68: 252–256
Bowden DM, Jones ML (1979) Aging research in nonhuman primates. In: Bowden DM (ed) Aging in nonhuman primates. Van Nostrand Reinhold, New York, pp 1–12
Card JP, Meade RP, Davis LG (1988) Immunocytochemical localization of the precursor protein for β-amyloid in the rat central nervous system. Neuron 1: 835–846
Castaño EM, Frangione B (1988) Biology of disease. Human amyloidosis, Alzheimer disease and related disorders. Lab Invest 58: 122–132
Dyrks T, Weidemann A, Multhaup G, Salbaum JM, Lemaire H-G, Kang J, Müller-Hill B, Masters CL, Beyreuther K (1988) Identification, transmembrane orientation and biogenesis of the amyloid A4 precursor of Alzheimer's disease. EMBO J 7: 949–957
Glenner GG (1979) Congophilic microangiopathy in the pathogenesis of Alzheimer's syndrome (presenile dementia). Med Hypotheses 5: 1231–1236
Goedert M (1987) Neuronal localization of amyloid beta protein precursor mRNA in normal human brain and in Alzheimer's disease. EMBO J 6: 3627–3632
Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC (1987) Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science 235: 877–880
Hart MN, Merz P, Bennett-Gray J, Menezes AH, Goeken JA, Schelper RL, Wisniewski HM (1988) β-Amyloid protein of Alzheimer's disease is found in cerebral and spinal cord vascular malformations. Am J Pathol 132: 167–172
Higgins GA, Lewis DA, Bahmanyar S, Goldgaber D, Gajdusek DC, Young WG, Morrison JH, Wilson MC, (1988) Differential regulation of amyloid-β-protein mRNA expression within hippocampal neuronal subpopulations in Alzheimer disease. Proc Natl Acad Sci USA 85: 1297–1301
Kang J, Lemaire H-G, Unterbeck A, Salbaum JM, Masters CL, Grzeschik K-H, Multhaup G, Beyreuther K, Müller-Hill B (1987) The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736
Kemper T (1984) Neuroanatomical and neuropathological changes in normal aging and in dementia. In: Albert ML (ed) Clinical neurology of aging. Oxford University Press, New York, pp 9–52
Koo EH, Goldgaber D, Sisodia SS, Applegate MD, Gajdusek DC, Price DL (1988) Studies of β-amyloid precursor gene expression in brains of aged monkeys. Soc Neurosci Abstr 14 637
Koo EH, Sisodia SS, Archer DR, Martin LJ, Beyreuther K, Weidemann A, Price DL (1989) Amyloid precursor protein (APP) undergoes fast anterograde transport. Soc Neurosci Abstr 15: 23
Lewis DA, Higgins GA, Young WG, Goldgaber D, Gajdusek DC, Wilson MC, Morrison JH (1988) Distribution of precursor amyloid-β-protein messenger RNA in human cerebral cortex: relationship to neurofibrillary tangles and neuritic plaques. Proc Natl Acad Sci USA 85: 1691–1695
Life (1959) Able and Baker, U.S. heroes, come back from space. 46: 38–40
Mandybur TI (1975) The incidence of cerebral amyloid angiopathy in Alzheimer's disease. Neurology 25: 120–126
Masters CL, Multhaup, G, Simms G, Pottgiesser J, Martins RN, Beyreuther K (1985) Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J 4: 2757–2763
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 82: 4245–4249
Miyakawa T, Shimoji A, Kuramoto R, Higuchi Y (1982) The relationship between senile plaques and cerebral blood vessels in Alzheimer's disease and senile dementia Morphological mechanism of senile plaque production. Virchows Arch [B] 40: 121–129
Miyakawa T, Katsuragi S, Watanabe K, Shimoji, A, Ikeuchi Y (1986) Ultrastructural studies of amyloid fibrils and senile plaques in human brain. Acta Neuropathol (Berl) 70: 202–208
Morimatsu M, Hirai S, Muramatsu A, Yoshikawa M (1975) Senile degenerative brain lesions and dementia. J Am Geriatr Soc 23: 390–406
Mountjoy CQ, Tomlinson BE, Gibson RH (1982) Amyloid and senile plaques and cerebral blood vessels. A semi-quantitative investigation of a possible relationship. J Neurol Sci 57: 89–103
Müller-Hill B, Beyreuther K (1989) Molecular biology of Alzheimer's disease. Annu Rev Biochem 58: 287–307
Palmert MR, Golde TE, Cohen ML, Kovacs DM, Tanzi RE, Gusella JF, Usiak MF, Younkin LH, Younkin SG (1988) Amyloid protein precursor messenger, RNAs: differential expression in Alzheimer's disease. Science 241: 1080–1084
Peterson EW, Schulz DM (1961) Amyloid in vessels of a vascular malformation in brain. Arch Pathol 72: 480–483
Ponte P, Gonzalez-DeWhitt P, Schilling J, Miller J, Hsu D, Greenberg B, Davis K, Wallace W, Lieberburg I, Fuller F, Cordell B (1988) A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature 331: 525–527
Powers JM, Schlaepfer WW, Willingham MC, Hall BJ (1981) An immunoperoxidase study of senile cerebral amyloidosis with pathogenetic considerations. J Neuropathol Exp Neurol 40: 592–612
Prelli F, Castaño E, Glenner GG, Frangione B (1988) Differences between vascular and plaque core amyloid in Alzheimer's disease. J Neurochem 51: 648–651
Price DL (1986) New perspectives on Alzheimer's disease. Annu Rev Neurosci 9: 489–512
Price DL, Koo EH, Sisodia SS, Martin LJ, Walker LC, Kitt CA, Cork LC (1990) Brain abnormalities in aged monkeys: a model sharing features with alzheimer's disease. In: Maurer K, Riederer P Beckmann H (eds) Alzheimer's disease: epidemiology, neuropathology, neurochemistry, clinics, Springer, Wien (in press)
Robakis NK, Ramakrishna N, Wolfe G, Wisniewski HM (1987) Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci USA 84: 4190–4194
Rosenblum LA, Coe CL (1985) Handbook of squirrel monkey research. Plenum, New York
Scholz W (1938) Studien zur Pathologie der Hirngefäße. II. Die drüsige Entartung der Hirnarterien und-capillaren. Z Ges Neurol Psychiatr 162:692–715
Selkoe DJ (1986) Altered structural proteins in plaques and tangles: what do they tell us about the biology of Alzheimer's disease? Neurobiol Aging 7: 425–432
Selkoe DJ (1989) Biochemistry of altered brain proteins in Alzheimer's disease. Annu Rev Neurosci 12: 463–490
Selkoe DJ, Bell DS, Podlisny MB, Price DL, Cork LC (1987) conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease Science 235: 873–877
Shivers BD, Hilbich C, Multhaup G, Salbaum M, Beyreuther K, Seeburg PH (1988) Alzheimer's disease amyloidogenic glycoprotein: expression pattern in rat brain suggests a role in cell contact. EMBO J 7: 1365–1370
Sternberger LA (1979) Immunocytochemistry 2nd edn. Wiley, New York
Sternberger LA, Sternberger NH (1983) Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA 80: 6126–6130
Tagliavini F, Giaccone G, Frangione B, Bugiani O (1988) Preamyloid deposits in the cerebral cortex of patients with Alzheimer's disease and nondemented individuals. Neurosci Lett. 93: 191–196
Tanzi RE, Gusella JF, Watkins PC, Bruns GAP, St George-Hyslop P, Van Keuren ML, Patterson D, Pagan S, Kurnit DM, Neve RL (1987) Amyloid β protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science 235: 880–884
Tomonaga M (1981) Cerebral amyloid angiopathy in the elderly. J Am Geriatr Soc 29: 151–157
Ulrich J (1985) Alzheimer changes in nondemented patients younger than sixty-five: possible early stages of Alzheimer's disease and senile dementia of Alzheimer type. Ann Neurol 17: 273–277
van Duinen SG, Castaño EM, Prelli F, Bots GTAB, Luyendijk W, Frangione B (1987) Hereditary cerebral hemorrhage with amyloidosis in patients of Dutch origin is related to Alzheimer's disease. Proc Natl Acad Sci USA 84: 5991–5994
Vinters HV (1987) Cerebral amyloid angiopathy A critical review. Stroke 18: 311–324
Vinters HV, Gilbert JJ (1983) Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke 14:924–928
Vinters HV, Miller BL, Pardridge WM (1988) Brain amyloid and Alzheimer disease. Ann Intern Med 109: 41–54
Walker LC, Kitt CA, Schwam E, Buckwald B, Garcia F, Sepinwall J, Price DL (1987) Senile plaques in aged squirrel monkeys. Neurobiol Aging 8: 291–296
Weidemann A, König G, Bunke D, Fischer P, Salbaum JM, Masters CL, Beyreuther K (1989) Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell 57: 115–126
Wisniewski HM, Ghetti B, Terry RD (1973) Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J Neuropathol Exp Neurol 32: 566–584
Wong CW, Quaranta V, Glenner GG (1985) Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci USA 82: 8729–8732
Yamaguchi H, Hirai S, Morimatsu M, Shoji M, Harigaya Y (1988) Diffuse type of senile plaques in the brains of Alzheimer-type dementia. Acta Neuropathol 77: 113–119
Yamaguchi H, Hirai S, Morimatsu M, Shoji M, Ihara Y (1988) A variety of cerebral amyloid deposits in the brains of the Alzheimer-type demetia demonstrated by β protein immunostaining. Acta Neuropathol 76: 541–549
Author information
Authors and Affiliations
Additional information
Supported by grants from the U.S.Public Health Service (NIH NS 20471, AG05146). Dr.Price is the recipient of a Leadership and Excellence in Alzheimer's Disease (LEAD) award (NIA AG07914) and a Javits Neuroscience Investigator Award (NIH NS 10580)
Rights and permissions
About this article
Cite this article
Walker, L.C., Masters, C., Beyreuther, K. et al. Amyloid in the brains of aged squirrel monkeys. Acta Neuropathol 80, 381–387 (1990). https://doi.org/10.1007/BF00307691
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00307691