Abstract
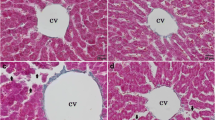
The protective effect of selenite, seleno-dl-methionine and biological selenium against the renotoxicity of mercury was tested in rats. As the source of biological selenium, the liver soluble fraction of rats given 60 μmoles/kg selenite 3 days before sacrifice was used. The aim of the experiments was to test whether protective efficiency follows the reported order of ability to form HgSe. Mercury was given subcutaneously in doses of 2.5, 5.0 and 7.5 μmoles/kg HgCl2 and selenium was given in equimolar doses at the same time as Hg2+. Liver soluble fraction, biological selenium or liver soluble fraction supplemented with selenite or seleno-dl-methionine were given orally, while in experiments without liver soluble fraction the two selenium compounds were given subcutaneously. Biological selenium was tested only at the two lower dose levels. Both biological selenium and seleno-dl-methionine decreased the urinary excretion of mercury in the first 48 h, but less so than selenite and only selenite decreased the renal content of mercury at the end of this period. Urinary alkaline phosphatase activity and plasma urea nitrogen at the 2.5 and 5.0 μmoles/kg dose levels decreased in the order of no selenium > biological selenium > seleno-dl-methionine > selenite. As the reported HgSe formation increases in the same order, the experiments support the role of HgSe formation in the protective effect. The degree of necrotic damage in the P2 and P3 regions of the proximal tubular cells increased in the same order as the biochemical indicators at the 5.0 and 7.5 μmoles/kg dose levels. Necrotic damage at the lower dose level of mercury was slight and differences between groups could be explained by random distribution.
Similar content being viewed by others
References
Braun JP, Rico AG, Benard P, Burgat-Sacaze V, Eghbali B, Godfrain JC (1978) La gamma-glutamyl transferase urinaire en toxicologie renale chez le rat. Bases de son utilization-interet lors de nephrite aigue mercurielle. Toxicology 11: 73–82
Burk RF, Foster KA, Greenfield PM, Kiker KW (1974) Binding of simultaneously administered inorganic selenium and mercury to a rat plasma protein. Proc Soc Exp Biol Med 145: 782–785
Chmielnicka J, Komsta-Szumska E (1980) Variation of the level of mercury and metallothionein in the kidneys and liver of rats with time of exposure to sodium selenite. Biol Trace Element Res 2: 109–120
Chmielnicka J, Breznicka E, Snieady A (1986) Kidney concentrations and urinary excretion of mercury, zinc and copper following the administration of mercuric chloride and sodium selenite to rats. Arch Toxicol 59: 16–20
Eybl V, Sykora J, Mertl F (1969) Einfluß von Natriumselenit, Natriumtellurit und Natriumsulfit auf Retention und Verteilung von Quecksilber bei Mäusen. Arch Toxicol 25: 296–305
Gasiewicz TA, Smith JC (1978) Properties of the cadmium and selenium complex formed in rat plasma in vivo and in vitro. Chem Biol Interact 23: 171–183
Groth DH, Stettler L, Mackay G (1976) Interaction of mercury cadmium, selenium, tellurium, arsenic and beryllium. In: Nordberg GF (ed) Effects and dose-response relationships of toxic metals. Elsevier, Amsterdam, pp 527–543
Harber MH, Jennings RB (1965) Renal response of rat to mercury. Arch Pathol 79: 218–222
Hoffman JL (1980) The rate of transmethylation in mouse liver as measured by trapping S-adenosylhomocysteine. Arch Biochem Biophys 205: 132–135
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin Phenol reagent. J Biol Chem 193: 265–275
Magos L, Webb M (1976) Differences in distribution and excretion of selenium and cadmium or mercury after their simultaneous administration subcutaneously in equimolar doses. Arch Toxicol 36: 63–69
Magos L, Webb M (1980) The interaction of selenium with cadmium and mercury. CRC Crit Rev Toxicol 8: 1–42
Magos L, Webb M, Hudson AR (1979) Complex formation between selenium and methylmercury. Chem Biol Interact 28: 359–362
Magos L, Clarkson TW, Hudson AR (1984a) Differences in the effects of selenite and biological selenium on the chemical form and distribution of mercury after the simultaneous administration of HgCl2 and selenium to rats. J Pharm Exp Ther 228: 478–483
Magos L, Sparrow S, Snowden RT (1984b) Effect of prolonged saline loading on HgCl2-induced renal tubular damage. Br J Exp Pathol 65: 567–575
McConnel KP, Roth DM (1966) Respiratory excretion of selenium. Proc Soc Exp Biol Med 123: 919–921
Naganuma A, Imura N (1981) Properties of mercury and selenium in a high molecular weight substance in rabbit tissues formed by simultaneous administration. Pharmacol Biochem Behav 15: 449–454
Page EB (1963) Ordered hypotheses for multiple treatments: significance test for linear ranks. Am Statist Assoc J 58: 215–230
Parizek J, Ostadalova I (1967) The protective effect of small amounts of selenite in sublimate intoxication. Experientia 23: 142–143
Snedecor GW, Cochrane WG (1980) Statistical methods, 7th Edition. The Iowa State University Press, Iowa, USA
Tandon SK, Magos L, Webb M (1986) The stimulation and inhibition of the exhalation of volatile selenium. Biochem Pharmacol 35: 2763–2766
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Magos, L., Clarkson, T.W., Sparrow, S. et al. Comparison of the protection given by selenite, selenomethionine and biological selenium against the renotoxicity of mercury. Arch Toxicol 60, 422–426 (1987). https://doi.org/10.1007/BF00302384
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00302384