Abstract
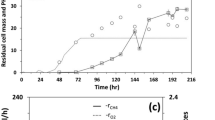
The production of biomass, polysaccharide storage material and H2 from malate was studied in the wild-type and mutants RdcI, RdcII and RdcI/cII of Rhodobacter capsulatus. The mutants are defective in either copy I, copy II or both copies of the nitrogenase genes nifA and nifB. Stationary phase levels of biomass, polysaccharide and H2 were determined in phototrophic batch cultures grown with 30 mM of d,l-malate and either 2, 5, or 8 mM of ammonium or 7 mM of glutamate. Calculation of the amounts of malate converted into the three products revealed that, at 8 mM of ammonium and 7 mM of glutamate, malate consumption and product formation were balanced. But with decreasing ammonium concentrations malate not converted into biomass was utilized with decreasing efficiency in polysaccharide and H2 formation. This suggests formation of unknown products at the lower ammonium concentrations. Under conditions of optimal N supply, 80% of the malate not used for biomass production was converted by the wild-type and strain RdcII to H2 and CO2. Mutant RdcI exhibited slightly decreased H2 production. The double mutant did not evolve H2 but accumulated increased amounts of polysaccharide. However, the amounts of polysaccharide were lower than should be expected if all of the spare malate, not utilized by the double mutant for H2 production, was converted into storage material. This and incomplete conversion of malate into known products at low ammonium supplies suggests that polysaccharide accumulation does not compete with the process of H2 formation for malate.
Similar content being viewed by others
References
Boogerd FC, van Versefeld HW, Torenfliet D, Brester M, Stouthamer AH (1984) Reconsideration of the efficiency of energy transduction in Paracoccus denitrificans during growth under a variety of culture conditions. Arch Microbiol 139: 344–350
Dawes EA, Senior PJ (1973) The role and regulation of energy reserve polymers. Adv Microb Physiol 10: 135–266
Haaker H, Laane C, Hellingwerf K, Houwer B, Konings WN, Veeger C (1982) Short-term regulation of the nitrogenase activity in Rhodopseudomonas sphaeroides. Eur J Biochem 127: 639–645
Hallenbeck PC (1987) Molecular aspects of nitrogen fixation by photosynthetic prokaryotes. Crit Rev Microbiol 14: 1–14
Herbert D, Phipps PJ, Strange RE (1971) Chemical analysis of microbial cells. Methods Microbiol 5B: 209–344
Hillmer P, Gest H (1977) H2 metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata: H2 production by growing cultures. J Bacteriol 129: 724–731
Klipp W, Masepohl B, Pühler A (1988) Identification and mapping of nitrogen fixation genes of Rhodobacter capsulatus: duplication of a nifA-nifB region. J Bacteriol 170: 693–699
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
Masepohl B, Klipp W, Pühler A (1988) Genetic characterization and sequence analysis of the duplicated nifA/nifB gene region of Rhodobacter capsulatus. Mol Gen Genet 212: 27–37
Odom JM, Wall JD (1983) Photoproduction of H2 from cellulose by an anaerobic bacterial culture. Appl Environ Microbiol 42: 1300–1305
Ormerod JG, Ormerod KS, Gest H (1961) Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationship with nitrogen metabolism. Arch Biochem Biophys 94: 449–463
Pirt SJ (1982) Maintenance energy: a general model for energy-limited and energy-sufficient growth. Arch Microbiol 133: 300–302
Stanier RY, Doudoroff M, Kunisawa R, Contopoulou R (1959) The role of organic substrates in bacterial photosynthesis. Proc Natl Acad Sci USA 45: 1246–1260
Takakuwa S, Odom JM, Wall JD (1983) Hydrogen uptake deficient mutants of Rhodopseudomonas capsulata. Arch Microbiol 136: 20–25
Tempest DM, Neijssel OW (1984) The status of YATP and maintenance energy as biologically interpretable phenomena. Annu Rev Microbiol 38: 459–486
Vignais PM, Colbeau A, Willison JC, Jouanneau Y (1985) Hydrogenase, nitrogenase, and hydrogen metabolism in the photosynthetic bacteria. Adv Microb Physiol 26: 156–234
Watts Pirt M, Pirt SJ (1977) Photosynthetic production of biomass and starch by Chlorella in chemostat culture. J Appl Chem Biotechnol 27: 643–650
Weaver PF, Wall JD, Gest H (1975) Characterization of Rhodopseudomonas capsulata. Arch Microbiol 105: 207–216
Willison JC, Madern D, Vignais PM (1984) Increased photoproduction of hydrogen by non-autotrophic mutants of Rhodopseudomonas capsulata. Biochem J 219: 593–600
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Klein, G., Klipp, W., Jahn, A. et al. The relationship of biomass, polysaccharide and H2 formation in the wild-type and nifA/nifB mutants of Rhodobacter capsulatus . Arch. Microbiol. 155, 477–482 (1991). https://doi.org/10.1007/BF00244965
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00244965