Summary
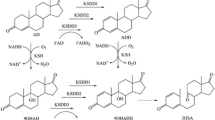
Stable mutants showing improved 11-hydroxylation of Substance S were isolated, following treatment with N-methyl-N′-nitro-N-nitrosuguanidine (NTG) and regeneration of uninucleate protoplasts of the appropriate fungal strains. This procedure was especially suitable for obtaining more directed 11β-hydroxylation of Substance S with Curvularia lunata IM 2901. Apart from producing cortisol (11β-hydroxy-S), the parent strain formed several by-products that significantly lowered the yield of the desired 11β-hydroxyderivative. Isolated mutants of this microoraganism carried out directed 11β-hydroxylation with only a small amount of one of the by-products, which resulted in a much higher yield of cortisol.
Similar content being viewed by others
References
Colingsworth DR, Karnemaat JN, Hanson FR, Brunner MP, Mann CK, Haines WJ (1953) A partial microbiological synthesis of hydrocortisone. J Biol Chem 203:807–813
Dermastia M, Rozman D, Komel R (1991) Heterologous transformation of Cochliobolus lunatus. FEMS Microbiol Lett 77:145–150
DeVries OMH, Wessels JGH (1972) Release of protoplasts from Schizophyllum commune by a lytic enzyme preparation from Trichoderma viride. J Gen Microbiol 73:13–22
Długónski J, Sedlaczek L, Jaworski A (1984) Protoplast release from fungi capable of steroid transformation. Can J Microbiol 30:57–62
Filippini S, Garofano L, Breme U (1986) Nitrozoguanidine mutagenesis on Streptomyces fradiae protoplasts. Fifth International Symposium on the Genetics of Industrial Microorganisms, GIM 86. Book of Abstracts, Split, Yugoslavia, September 14–20, p 106
Gaugy D, Fevre M (1982) Protoplast production from Saprolegnia monoica. Microbios 34:89–98
Hesselink P (1988) Sterol side chain cleavage by Mycobacterium. Characterization, optimization and genetics. PhD thesis, University of Groningen, The Netherlands
Jekkel A, Csajagi E, Ilköy E, Ambrus G (1989) Genetic recombination by spheroplast fusion on sterol-transforming Mycobacterium strains. J Gen Microbiol 135:1727–1733
Keller U (1983) Highly efficient mutagenesis of Claviceps purpurea by using protoplasts. Appl Environ Microbiol 46:580–584
Lin YY, Smith LL (1970) Microbial hydroxylations. VII. Kinetic studies on the hydroxylation of 19-norsteroids by Curvularia lunata. Biochim Biophys Acta 218:515–525
Malina H, Tempete C, Robert-Gero M (1985) Enhanced sinefungin production by medium improvement, mutagenesis, and protoplasts regeneration of Streptomyces incarnatus NRRL 8089. J Antibiot 38:1204–1210
Matěju J, Maršalkova J, Nohynek M, Steinerova N (1991) Mutant strains of Streptomyces cinnamonensis protoplasts. Cultural and physiological conditions. Folia Microbiol 36:42–48
Osiewacz HD, Weber A (1989) DNA mediated transformation of the filamentous fungus Curvularia lunata using a dominant selectable marker. Appl Microbiol Biotechnol 30:375–380
Peppler HJ, Perlman D (1979) Microbial technology. Academic Press, New York, pp22, 24
Prescott SC, Dunn CC (1959) Industrial microbiology. McGraw-Hill, New York, p 519
Rowlands AR (1983) Industrial fungal genetics and strain improvement. In: Smith JE, Berry DR (eds) The filamentousfungi, vol 4. Fungal technology. Arnold, London, p346
Sedlaczek L (1988) Biotransformations of steroids. CRC Crit Rev Biotechnol 7:187–236
Sedlaczek L, Jaworski A, Wilmańska D (1981) Transformation of steroids by fungal spores. I. Chemical changes of Cunninghamella elegans spores and mycelium during cortexolone hydroxylation. Eur J Appl Microbiol Biotechnol 13:155–160
Sedlaczek L, Wilmańska D, Długoński J, Uszycka-Horawa T, Budzyńska M, Skibińska M, Jaworska R, Trzcińska Z, Szczepaniak J. Goźlińska H (1985) Polish patent no. 123 535
Shull GM, Kita DA (1955) Microbiological conversion of steroids. I. Introduction of 11β-hydroxyl group into C21 steroids. J Am Chem Soc 77:763
Smith LL (1984) Steroids. In: Rehm H-J, Reed G (eds) Biotechnology, a comprehensive treatise in 8 volumes, vol 6a. Verlag Chemie, Weinheim, p33
Author information
Authors and Affiliations
Additional information
Correspondence to: L. Sedlaczek
Rights and permissions
About this article
Cite this article
Wilmańska, D., Milczarek, K., Rumijowska, A. et al. Elimination of by-products in 11β-hydroxylation of Substance S using Curvularia lunata clones regenerated from NTG-treated protoplasts. Appl Microbiol Biotechnol 37, 626–630 (1992). https://doi.org/10.1007/BF00240738
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00240738