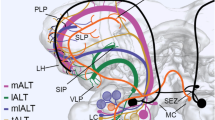
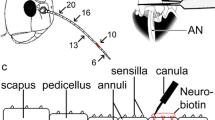
Abstract
A prominent hypothesis for the function of the glomerular structures in the primary olfactory neuropil of many groups of vertebrate and invertebrate animals is that they enable the processing and coding of information about the chemical compounds that compose complex odors. Previous studies have indicated that various degrees of glomerulus formation in the antennal lobes of the brain of the moth Manduca sexta can be effected by reducing the number of olfactory sensory axons that grow from the antenna into the antennal lobe during metamorphosis. To test the hypothesis that the presence of glomerular structure is necessary to process and identify odors, we substantially reduced, by surgery, the number of antennal segments in developing moths and upon metamorphosis we observed and quantified behavioral responses known to be elicited by odors. Intact and lesioned adult female moths were challenged to fly upwind to the source of an attractive host-plant odor in a wind tunnel. Some of the moths that had developed with reduced olfactory input flew upwind to the odor source. The flight behavior of these individuals was similar to the odor-mediated flight typically observed in moths that had developed normally. Histological analysis of the moths’ antennal lobes revealed that the lobes of more than half of the respondents that had been lesioned during development lacked normal glomerular organization. The neuropil of these abnormally developed antennal lobes was mostly aglomerular, but with a few isolated, clearly abnormal glomerulus-like structures. This suggests either that even a few abnormal glomeruli are sufficient to mediate this specific behavior or that “canonical” glomerular organization per se is not necessary for this odor-mediated behavior.
Similar content being viewed by others
References
Ache BW (1991) Phytogeny of taste and smell. In: Getchell TV, Doty RL, Bartoshuk LM, Snow JB Jr (eds), Smell and taste in health and disease. Raven Press, New York, pp 3–18
Arbas EA, Willis MA, Kanzaki R (1993) Organization of a goaloriented locomotion: pheromone-modulated flight behavior of moths. In: Beer R, Ritzmann R, McKenna T (eds) Biological neural networks in invertebrate neuroethology and robotics. Academic Press, New York, pp 159–198
Baier H, Korsching S (1994) Olfactory glomeruli in the zebrafish form an invariant pattern and are identifiable across animals. J Neurosci 14: 219–230
Bailey MS, Poston MR, Shipley MT (1989) Unique morphology of olfactory bulb radial glia: role in glomerular development? Soc Neurosci Abstr 15: 690
Bell RA, Joachim FA (1976) Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann Entomol Soc Am 69: 365–373
Boeckh J, Distler P, Ernst KD, Hosl M, Malun D (1990) Olfactory bulb and antennal lobe. In: Schild D (ed) Chemosensory information processing. NATO ASI series H 39, Springer, Berlin Heidelberg, pp 201–227
Buonviso N, Chaput MA (1990) Response similarity to odors in olfactory bulb output cells presumed to be connected to the same glomerulus: electrophysiological study using simultaneous single-unit recordings. J Neurophysiol 63: 447–455
Büschges A., Ramirez J-M, Pearson KG (1992) Reorganization of sensory regulation of locust flight after partial deafferentation. J Neurobiol 23: 31–43
Camazine SM, Hildebrand JG (1979) Central projections of antennal sensory neurons in mature and developing Manduca sexta. Soc Neurosci Abstr 5: 155
Chase R (1985) Responses to odors mapped in snail tentacle and brain by [14C]-2-deoxyglucose autoradiography. J Neurosci 5: 2930–2939
Dethier VG (1990) Five hundred million years of olfaction. In: Colbow K (ed) R.H. Wright Lectures on Olfaction. Simon Fraser University, Burnaby BC Canada, pp 1–37
Graziadei PPC, Monti-Graziadei GA (1992) The influence of the olfactory placode on the development of the telencephalon in Xenopus laevis. Neuroscience 46: 612–629
Hildebrand JG, Hall LM, Osmond BC (1979) Distributin of binding sites for 125I-labeled α-bungarotoxin in normal and deafferented antennal lobes of Manduca sexta. Proc Natl Acad Sci USA 76: 499–503
Homberg U, Christensen TC, Hildebrand JG (1989) Structure and function of the deutocerebrum in insects. Annu Rev Entomol 34: 477–501
Hudson R, Distel H (1987) Regional autonomy in the peripheral processing of odor signals in newborn rabbits. Brain Res 421: 85–94
Hudson R, Distel H, Zippel HP (1990) Perceptual performance in peripherally reduced olfactory systems. In: Schild D (ed) Chemosensory information processing. NATO ASI series H 39, Springer, Berlin Heidelberg, pp 259–269
Jastreboff PJ, Pedersen PE, Greer CA, Stewart WB, Kauer JS, Benson TE, Shepherd GM (1984) Specific olfactory receptor populations projecting to identified glomeruli in the rat olfactory bulb. Proc Natl Acad Sci USA 81: 5250–5254
Jourdan F, Duveau L, Astic L, Holley A (1980) A spatial distribution of [14C]2-deoxyglucose uptake in the olfactory bulbs of rats stimulated with two different odors. Brain Res 188: 139–154
Kauer JS (1987) Coding in the olfactory system. In: Finger TE, Silver WL (eds) Neurobiology of taste and smell. John Wiley and Sons, New York, pp 205–231
Kauer JS (1991) Contributions of topography and parallel processing to odor coding in the vertebrate olfactory pathway. Trends Neurosci 14: 79–85
Kauer JS, Senseman DN, Cohen LB (1987) Odor-elicited activity monitored simultaneously from 124 regions of the salamander olfactory bulb using a voltage-sensitive dye. Brain Res 418: 255–261
Kent KS (1985) Metamorphosis of the antennal center and the influence of sensory innervation on the formation of glomeruli in the hawk moth Manduca sexta. PhD dissertation, Harvard University, Cambridge
Kent KS, Harrow ID, Quartararo P, Hildebrand JG (1986) An accessory olfactory pathway in Lepidoptera: the labial pit organ and its central projections in Manduca sexta and certain other sphinx moths and silk moths. Cell Tissue Res 245: 237–245
Lancet D, Greer CA, Kauer JS, Shepherd GM (1982) Mapping of odor-related neuronal activity in the olfactory bulb by highresolution 2-deoxyglucose autoradiography. Proc Natl Acad Sci USA 79: 670–674
Lee J-K, Strausfeld NJ (1990) Structure, distribution and number of surface sensilla and their receptor cells on the olfactory appendage of the male moth Manduca sexta. J Neurocytol 19: 519–538
Miller JR, Strickler KL (1984) Finding and accepting host plants. In: Bell WJ and Cardé RT (eds) Chemical ecology of insects. Chapman and Hall Ltd, London, pp 127–157
Mori K, Malaga N, Imamura K (1992) Differential specificities of single mitral cells in rabbit olfactory bulb for a homologous series of fatty acid odor molecules. J Neurophysiol 67: 786–789
Niehaus M (1981) Flight and flight control by the antennae in the small tortoiseshell (Aglais urticae L., Lepidoptera) II. Flight mill and tree flight experiments. J Comp Physiol 145: 257–264
Oland LA, Tolbert LP (1987) Glial patterns during early development of antennal lobes of Manduca sexta: a comparison between normal lobes and lobes deprived of antennal axons. J Comp Neurol 255: 196–207
Oland LA, Tolbert LP (1988) Effects of hydroxyurea parallel the effects of radiation in developing glomeruli in insects. J Comp Neurol 278: 377–387
Oland LA, Orr G, Tolbert LP (1990) Construction of a protoglomerular template by olfactory axons initiates the formation of olfactory glomeruli in the insect brain. J Neurosci 10: 2096–2112
Ramaswamy SB (1988) Host finding by moths: sensory modalities and behaviours. J Insect Physiol 34: 235–249
Ramón y Cajal S (1911) Histologie du système nerveux de l'homme et des vertebrés. Madrid:Istituto Ramón y Cajal, Vol II (1972 reprint), pp 647–674
Risser JM, Slotnick BM (1987) Suckling behavior in rat pups with lesions which destroy the modified glomerular complex. Brain Res Bull 19: 275–281
Rodrigues V (1988) Spatial coding of olfactory information in the antennal lobe of Drosophila melanogaster. Brain Res 453: 299–307
Rodrigues V, Buchner E (1984) [3H]2-Deoxyglucose mapping of odor-induced neuronal activity in the antennal lobes of Drosophila melanogaster. Brain Res 324: 374–378
Rospars J-P, Chambille I (1989) Identified glomeruli in the antennal lobes of insects: Invariance, sexual variation and postembryonic development. In: Singh RN, Strausfeld NJ (eds) Neurobiology of sensory systems, Plenum Press, New York, pp 319–353
Rospars J-P, Hildebrand JG (1992) Anatomical identification of glomeruli in the antennal lobes of the male sphinx moth Manduca sexta. Cell Tissue Res 270: 205–227
Ryan TA (1960) Significance tests for multiple comparison of proportions, variances and other statistics. Psychol Bull 46: 383–392
Sanes JR, Hildebrand JG (1976) Origin and morphogenesis of sensory neurons in an insect antenna. Dev Biol 51: 300–316
Sanes JR, Prescott DJ, Hildebrand JG (1977) Cholinergic neurochemical development of normal and deafferented antennal lobes during metamorphosis of the moth, Manduca sexta. Brain Res 119: 389–402
Sasaki M, Riddiford LM (1984) Regulation of reproductive behaviour and egg maturation in the tobacco hawk moth, Manduca sexta. Physiol Entomol 9: 315–327
Scott JW, Harrison T (1987) The olfactory bulb: anatomy and physiology. In: Northcutt G (ed) Neurobiology of taste and smell. John Wiley, New York, pp 151–178
Sharp FR, Kauer JS, Shepherd GM (1975) Local sites of activity-related glucose metabolism in rat olfactory bulb during olfactory stimulation. Brain Res 98: 596–600
Sharp FR, Kauer JS, Shepherd GM (1977) Laminar analysis of 2-deoxyglucose uptake in olfactory bulb and olfactory cortex of rabbit and rat. J Neurophysiol 40: 800–813
Shepherd GM (1972) Synaptic organization of the mammalian olfactory bulb. Physiol Rev 52: 864–917
Shepherd GM (1981) The olfactory glomerulus: its significance for sensory processing. In: Katsuki Y, Norgren R, Sato M (eds) Brain mechanisms of sensation. John Wiley, New York, pp 209–223
Shepherd GM (1990) Contribution toward a theory of olfaction. In: Colbow K (ed) RH Wright Lectures on Olfaction. Simon Fraser University, Burnaby BC Canada, pp 61–109
Skeen LC (1977) Odor-induced patterns of deoxyglucose consumption in the olfactory bulb of the tree shrew Tupaia glis. Brain Res 124: 147–153
Slotnick BM, Graham S, Laing DG, Bell GA (1987) Detection of propionic acid vapor by rats with lesions of olfactory bulb areas associated with high 2-DG uptake. Brain Res 417: 343–346
Stange G (1992) High resolution measurement of atmospheric carbon dioxide concentration changes by the labial palp organ of the moth Heliothis armigera (Lepidoptera: Noctuidae). J Comp Physiol A 171: 317–324
Stewart WB, Kauer JS, Shepherd GM (1979) Functional organization of rat olfactory bulb, analyzed by the 2-deoxyglucose method. J Comp Neurol 185: 715–734
Strausfeld NJ (1989) Insect vision and olfaction: common design principles of organization. In: Singh RN, Strausfeld NJ (eds) Neurobiology of sensory systems, Plenum Press, New York, pp 319–353
Tolbert LP (1989) Afferent axons from the antenna influence the number and placement of intrinsic synapses in the antennal lobes of Manduca sexta. Synapse 3: 83–95
Tolbert LP, Sirianni PA (1990) Requirement for olfactory axons in the induction and stabilization of olfactory glomeruli in an insect. J Comp Neurol 298: 69–82
Vickers NJ, Baker TC (1991) The effects of unilateral antennectomy on the flight behavior of male Heliothis virescens in a pheromone plume. Physiol Entomol 16: 497–506
Waldrop BR, Harrow ID, Kovelman R, Hildebrand JG (1986) Olfactorily guided oviposition in the moth Manduca sexta: host-plant preference and induction. Soc Neurosci Abstr 12: 41
Willis MA, Arbas EA (1991) Odor-modulated upwind flight of the sphinx moth, Manduca sexta L. J Comp Physiol A 169: 427–440
Yamamoto RT, Jenkins RY (1972) Hostplant preferences of tobacco hornworm moths. In: Rodriquez JG (ed) Insect and mite nutrition. North-Holland, Amsterdam, pp 567–574
Yamamoto RT, Jenkins RY, McClusky RK (1969) Factors determining the selection of plants for oviposition by the tobacco hornworm Manduca sexta. Entomol Exp Appl 12: 504–508
Zippel HP, Meyer DL, Knaust M (1988) Peripheral and central post-lesion plasticity in the olfactory system of the goldfish: behavior and morphology. In: Flohr H (ed) Post-lesion neural plasticity. Springer, Berlin Heidelberg New York, pp 577–591
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Willis, M.A., Butler, M.A. & Tolbert, L.P. Normal glomerular organization of the antennal lobes is not necessary for odor-modulated flight in female moths. J Comp Physiol A 176, 205–216 (1995). https://doi.org/10.1007/BF00239923
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00239923