Summary
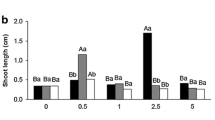
The promotive effect of ethylene inhibitors (Els), i.e. AgNO3 and aminoethoxyvinylglycine (AVG) on de novo shoot regeneration from cultured cotyledonary explants of Brassica campestris ssp. pekinensis cv. Shantung in relation to polyamines (PAs) was investigated. The endogenous levels of free putrescine and spermidine in the explant decreased sharply after 1–3 days of culture, whereas endogenous spermine increased, irrespective of the absence or presence of Els. AgNO3 at 30 μM did not affect endogenous PAs during two weeks of culture. In contrast, explants grown on medium containing 5 μM AVG produced higher levels of free putrescine and spermine which increased rapidly after three days and reached a peak at 10 days. An exogenous application of 5 mM putrescine also resulted in a similar surge of endogenous free spermine of the explant. More strikingly, shoot regeneration from explants grown in the presence of 1–20 mM putrescine, 0.1–2.5 mM spermidine, or 0.1–1 mM spermine was enhanced after three weeks of culture. However, exogenous PAs generally did not affect ethylene production, and endogenous levels of 1-aminocyclopropane-1-carboxylate (ACC) synthase activity and ACC of the explant. This study shows the PA requirement for shoot regeneration from cotyledons of B. campestris ssp. pekinensis in vitro, and also indicates that the promotive effect of PAs on regeneration may not be due to an inhibition of ethylene biosynthesis.
Similar content being viewed by others
Abbreviations
- PAs:
-
polyamines
- AVG:
-
aminoethoxyvinylglycine
- SAM:
-
S-adenosylmethionine
- ACC:
-
1-aminocyclopropane-1-carboxylate
- Els:
-
ethylene inhibitors
References
Apelbaum A, Burgoon AC, Anderson AD, Lieberman M, BenArie R, Mattoo Ak (1981) Polyamines inhibits biosynthesis of ethylene in higher-plant tissue and fruit protoplasts. Plant Physiol. 68: 453–456.
Beyer EM Jr (1976) A potent inhibitor of ethylene action in plants. Plant Physiol. 58: 268–271.
Biasi R, Bagni N, Cista G (1988) Endogenous polyamines in apples and their relationship in fruit set and fruit growth. Plant Physiol. 73: 201–205.
Chi G-L, Barfield DG, Sim G-E, Pua E-C (1990) Effect of AgNO3 and aminoethoxyvinylglycine on in vitro shoot and root organogenesis from seedling explants of recalcitrant Brassica genotypes. Plant Cell Rep. 9: 195–198.
Chi G-L, Pua E-C (1989) Ethylene inhibitors enhanced de novo shoot regeneration from cotyledons of Brassica campestris ssp. chinensis (Chinese cabbage) in vitro. Plant Sci. 64: 243–250.
Chi G-L, Pua E-C, Goh C-J (1991) Role of ethylene on de novo shoot regeneration from cotyledonary explants of Brassica campestris ssp. pekinensis (Lour) Olsson in vitro. Plant Physiol. 96: 178–183.
Downs CG, Lovell PH (1986) The effect of spermidine and putrescine on the senescence of cut carnation. Physiol. Plant. 66: 679–684.
Evans PT, Malmberg RL (1989) Do polyamines have role in plant development? Annu. Rev. Plant Physiol. Plant Mol. Biol. 40: 235–269.
Feirer RP, Mignon G, Litvay JD (1984) Arginine decarboxylase and polyamines required for embryogenesis in the wild carrot. Science 223: 1433–1435.
Feirer RP, Wann SR, Einspair DW (1985) The effects of spermidine synthesis inhibitors on in-vitro plant development. Plant Growth Reg. 3: 319–327.
Fienberg AA, Choi HH, Libich WP, Sung ZR (1984) Developmental regulation and polyamine metabolism and differentiation of carrot culture. Planta 162: 532–539.
Flores HE (1990) Polyamines and plant stress. In: Alscher RG, Cumming JR, Allen NS (eds) Stress Responses in Plants: Adaptation and Acclimation Mechanisms. Wiley-Liss, J. Wiley Sons Inc Publ, New York, pp 217–239.
Flores HE, Glaston AW (1982) Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 69: 701–706.
Galston AW, Sawhney RK (1990) Polyamines in plant physiology. Plant Physiol. 94: 406–410.
Gerats AGM, Kaye C, Collins C, Malmberg RL (1988) Polyamine level in Petunia genotypes with normal and abnormal floral morphologies. Plant Physiol. 86: 390–393.
Heby O, Persson L (1990) Molecular genetics of polyamine synthesis in eukaryotic cells. TIBS 15: 153–158.
Jarvis BC, Yasmin S, Coleman MT (1985) RNA and protein metabolism during adventitious root formation in stem cuttings of Phaseolus aureus cultivar berkin. Physiol. Plant. 64: 53–59.
Kaur-Sawhney R, Tiburcio AF, Galston AW (1988) Spermidine and flower-bud differentiation in thin layer explants of tobacco. Planta 173: 282–284.
Kuehn GD, Rodriguez-Garay B, Bagga S, Phillips GC (1990) Novel occurrence of uncommon polyamines in higher plants. Plant Physiol. 94: 855–857.
Kumar PP, Thorpe TA (1989) Putrescine metabolism in excised cotyledons of Pinus radiata cultured in vitro. Physiol. Plant. 76: 521–526.
Lentini Z, Mussel H, Mutschler MA, Earle ED (1988) Ethylene generation and reversal of ethylene effects during development in vitro rapid-cycling Brassica campestris L. Plant Sci. 54: 75–81.
Lizada MCC, Yang SF (1979) A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal. Biochem. 100: 140–145.
McKeon TA, Yang SF (1987) Biosynthesis and metabolism of ethylene. In: Davies PJ (ed) Plant Hormones and Their Role in Plant Growth and Development, Martinus Nijhoff Publ, Dordrecht, The Netherlands, pp 94–112.
Meijer EGM, Simmonds J (1988) Polyamine levels in relation to growth and somatic embryogenesis of Medicago sativa. J. Exp. Bot. 203: 787–794.
Meurer-Grimes B, Strack D, Wray V, Wiermann R (1989) Accumulation patterns of polyamines and hydroxycinnamic acid conjugates during morphogenesis of Corylus acellana. Z. Naturforsch. 44c: 635–640.
Montague M, Armstrong T, Jaworski E (1979) Polyamine metabolism in embryogenic cells of Daucus carota. II. Changes in arginine decarboxylase activity. Plant Physiol. 63: 341–345.
Muhitch MJ, Edwards LA, Fletcher JS (1983) Influence of diamines and polyamines on the senescence of plant suspension culture. Plant Cell Rep. 2: 82–84.
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15: 473–493.
Narasimhulu SB, Chopra VL (1988) Species specific shoot regeneration response of cotyledonary explants of Brassicas. Plant Cell Rep. 7: 104–106.
Palmer EE (1992) Enhanced shoot regeneration from Brassica campestris by silver nitrate. Plant Cell Rep. 11: 541–545.
Pua E-C (1990) Somatic embryogenesis and plant regeneration from hypocotyl protoplasts of Brassica juncea (L.) Czern & Coss. Plant Sci. 68: 231–238.
Pua E-C (1993) Cellular and molecular aspects of ethylene on plant morphogenesis of recalcitrant Brassica species in vitro. Bull. Bot. Acad. Sin. 34: 191–209.
Pua E-C, Chi G-L (1993) De novo shoot morphogenesis and plant growth of mustard (Brassica juncea) in vitro in relation to ethylene. Physiol. Plant. 88: 467–474.
Purnhauser L, Medgyesy M, Czeko PJ, Marton L (1987) Stimulation of shoot regeneration in Triticum aestivum and Nicotians plumbaginifolia Viv. tissue culture using the ethylene inhibitor AgNO3. Plant Cell Rep. 6: 1–4.
Reid MS (1987) Ethylene in plant growth and development, and senescence. In: Davies PJ (ed) Plant Hormones and Their Role in Plant Growth and Development, Martinus Nijhoff Publ, Dordrecht, The Netherlands, pp 267–279.
Roberts DR, Walker MA, Thompson JE, Dumbroff EB (1983) The effects of inhibitors of polyamines and ethylene biosynthesis on senescence, ethylene production and polyamine levels in cut carnation. Plant Cell Physiol. 25: 315–322.
Smith TA (1990) Plant polyamines — metabolism and function. In: Flores HE, Arteca RN, Shannon JC (eds) Polyamines and Ethylene: Biochemistry, Physiology and Interactions. American Society for Plant Physiologists, Maryland, pp 1–23.
Songstad DD, Armstrong CL, Peterson WL (1991) AgNO3 increases type II callus production from immature embryos of maize inbred B73 and its derivatives. Plant Cell Rep. 9: 699–702.
Suttle JC (1981) Effect of polyamines on ethylene production. Phytochemistry 20: 1477–1480.
Torrigiani P, Altamura MM, Pasqua G, Monacelli B, Serafini-Fracassini D, Bagni N (1987) Free and conjugated polyamines during de novo floral vegetative bud formation in thin cell layers of tobacco. Physiol. Plant. 70: 453–460.
Vain P, Flament P, Soudain P (1989) Role of ethylene on embryogenic callus initiation and regeneration in Zea mays. J. Plant Physiol. 135: 537–540.
Veen H, Overbeek JHM (1989) The action of silver thiosulfate in carnation petals. In: Clijsters H, de Proft M, Marcelle R, van Poucke M (eds) Biochemical and Physiological Aspects of Ethylene Production in Lower and Higher Plants. Kluwer Academic Publ, Dordrecht, The Netherlands, pp 109–117.
Wang CY, Kramer GF (1990) Effect of polyamine treatment on ethylene production of apples. In: Flores HE, Arteca RN, Shannon JC (eds) Polyamines and Ethylene: Biochemistry, Physiology and Interactions, American Society for Plant Physiologists, Maryland, pp 411–413.
Yang SF, Hoffman NE (1884) Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 35: 155–189.
Author information
Authors and Affiliations
Additional information
Communicated by J. J. Finer
Rights and permissions
About this article
Cite this article
Chi, GL., Lin, WS., Lee, J.E.E. et al. Role of polyamines on de novo shoot morphogenesis from cotyledons of Brassica campestris ssp. pekinensis (Lour) Olsson in vitro . Plant Cell Reports 13, 323–329 (1994). https://doi.org/10.1007/BF00232630
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00232630