Summary
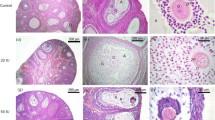
The development of granulosa-lutein cells was studied in 27 normal and 32 superovulated ewes between days 0–4 (day 0 began with the preovulatory LH peak in normal animals and the HCG injection in superovulated ewes). The pattern of differentiation was similar in both groups. Following initial hormonal stimulation (0–12 hours after LH or HCG), granulosa cells were approximately 100 μ2 and contained small, pleomorphic nuclei with large amounts of clumped chromatin. Elongate cells lining the basement membrane possessed large, heterogeneous dense bodies, and a well-developed Golgi apparatus. Mitotic figures were observed up to 6 hours prior to ovulation.
Sixteen to 20 hours following the LH surge or HCG injection, hypertrophy of granulosa cells was evident. Nuclei contained definitive nucleoli. Blood vessels in the theca interna were abundant and highly dilated.
Ovulation occurred approximately 24 hours after the LH peak or HCG injection. Visible signs of luteinization were evident 6–12 hours after ovulation. A slight increase in serum progesterone levels was detected.
The second post-ovulatory day was characterized by continuing hypertrophy of granulosa cells and extensive proliferation of smooth endoplasmic reticulum and mitochondria. Nuclei of granulosa cells were larger and possessed extremely large nucleoli. Numerous mitotic figures were apparent within the corpus luteum. Serum progesterone concentrations began increasing at 60–72 hours after hormone stimulation.
By the end of the third post-ovulatory day, the corpus luteum consisted of large, pleomorphic, parenchymal cells, interspersed between capillaries and connective tissue elements. Only an occasional mitotic figure was apparent within the corpus luteum at 100 hours.
Light microscopic autoradiography of 5, 10, and 15 day corpora lutea taken from ewes pulsed with 3H thymidine at specific times before and after ovulation revealed that granulosa cells did not undergo secondary mitoses following ovulation. In contrast, thecal, mesenchymal and endothelial cells did mitose on day 3.
Similar content being viewed by others
References
Abel, J.H., Jr., Verhage, H.G., McClellan, M.C., Niswender, G.D.: Ultrastructural analysis of the granulosa-luteal cell transition in the ovary of the dog. Cell Tiss. Res., 160, 155–176 (1975a)
Abel, J.H., Jr., McClellan, M.C., Verhage, H.G., Niswender, G.D.: Subcellular compartmentalization of the luteal cell in the ovary of the dog. Cell Tiss. Res., 158, 461–480 (1975b)
Adams, E.C., Hertig, A.T.: Studies on the human corpora lutea. I. Observations on the ultrastructure of development and regression of luteal cells during the menstrual cycle. J. Cell Biol. 41, 696–715 (1969a)
Adams, E.C., Hertig, A.T.: Studies on human corpora lutea. II. Observations on the ultrastructure of luteal cells during pregnancy. J. Cell Biol. 41, 716–735 (1969b)
Bennett, G., Leblond, C.P., Haddad, A.: Migration of glycoprotein from the Golgi apparatus to the surface of various cell types as shown by radioautography after labeled fucose injection into rats. J. Cell Biol. 60, 258–284 (1974)
Bjersing, L.: On the ultrastructure of granulosa lutein cells in porcine corpus luteum. Z. Zellforsch. 82, 187–211 (1967)
Bjersing, L., Hay, M.F., Moor, R.M., Short, R.V., Deane, H.W.: Endocrine activity, histochemistry and ultrastructure of ovine corpora lutea. I. Further observations on regression at the end of the oestrous cycle. Z. Zellforsch. 111, 437–457 (1970)
Bjersing, L., Hay, M.F., Kann, G., Moor, R.M., Naftolin, F., Scaramuzzi, R.J., Short, R.V., Younglai, E.V.: Changes in gonadotropins, ovarian steroids and follicular morphology in sheep at oestrus. J. Endocrin. 52, 465–479 (1972)
Bjorkman, N.: A study of the ultrastructure of the granulosa cells of the rat ovary. Acta Anat. 51, 125–147 (1962)
Blanchette, E.J.: Ovarian steroid cells. I. Differentiation of the lutein cells from the granulosa follicle cell during the preovulatory stage and under the influence of exogenous gonadotropins. J. Cell Biol. 31, 501–516 (1966a)
Blanchette, E.J.: Ovarian steroid cells. II. The lutein cell. J. Cell Biol. 31, 517–542 (1966a)
Cavazos, L.F., Anderson, L.L., Belt, W.D., Hendricks, D.M., Kraeling, R.R., Melampy, R.M.: Fine structure and progesterone levels in the corpus luteum of the pig during the estrous cycle. Biol. Reprod. 1, 83–106 (1969)
Christensen, K.: The Gonads (K.W. McKerns, ed.). The correlation of fine structure and function in steroid secreting cells, p. 415–488. New York: Appleton-Century-Crofts 1969
Crisp, T.M., Dessouky, D.A., Denys, F.R.: The fine structure of the human corpus luteum of early pregnancy and during the progestational phase of the menstrual cycle. Amer. J. Anat. 127, 37–70 (1970)
Crombie, P.R., Burton, R.D., Ackland, N.: The ultrastructure of the corpus luteum of the guinea pig. Z. Zellforsch. 115, 473–493 (1971)
Deane, H.W.: Intracellular lipids: their detection and significance. In: Frontiers in Cytology (S.L. Palay, ed.), p. 227–263. New York: Yale University Press 1958
Donaldson, L., Hansel, W.: Histological study of the bovine corpus luteum. J. Dairy Sci. 48, 905–909 (1965)
Enders, A.C.: Observations on the fine structure of lutein cells. J. Cell Biol. 12, 101–113 (1962)
Enders, A.: The cytology of the corpus luteum. Biol. Reprod. 8, 158–182 (1973)
Flint, A.P., Armstrong, D.T.: Dynamic aspects of ovarian cholesterol metabolism: Regulation by gonadotropins. In: Gonadotropins (B. Saxena, C. Beling, and H. Gandy, eds.), p. 271–186. New York: Wiley-Interscience 1972
Friend, D.S., Gilula, N.G.: A distinctive cell contact in the rat adrenal cortex. J. Cell Biol. 53, 148–163 (1972)
Friend, D.S., Farquhar, M.G.: Function of coated vesicles during protein absorption in the rat vas deferens. J. Cell Biol. 35, 357–376 (1967)
Gillim, S.W., Christensen, A.K., McLennan, C.E.: Fine structure of the human menstrual corpus luteum at its stage of maximum secretory activity. Amer. J. Anat. 126, 409–428 (1969)
Goodman, P., Latta, J.S., Wilson, R.B., Kadis, B.: The fine structure of sow lutein cells. Anat. Rec. 161, 77–90 (1968)
Guraya, S.S.: Morphology, histochemistry and biochemistry of human ovarian compartments and steroid hormone synthesis. Physio. Reviews 51, 785–807 (1971)
Hamilton, D.W., Jones, A.B., Fawcett, D.W.: Cholesterol biosynthesis in the mouse epididymis and ductus deferens: A biochemical and morphological study. Biol. Reprod. 1, 167–184 (1969)
Leavitt, W.W., Basom, C.R., Bagwell, J.N., Blaha, G.C.: Structure and function of hamster corpus luteum during the estrous cycle. Amer. J. Anat. 136, 235–249 (1973)
Lee, C.Y., Ryan, R.J.: Luteinizing hormone receptors in luteinized rat ovaries. In: Receptors for Reproductive Hormones (B. O'Malley, ed.), p. 419–430. New York: 1973
Lipner, H., Cross, N.L.: Morphology of the membrana granulosa of the ovarian follicle. Endocrin. 82, 638–641 (1968)
Niswender, G.D., Midgley, A.R., Monroe, S.E., Reichert, L.E., Jr.: Radioimmunoassay for rat luteinizing hormone with antiovine LH serum and ovine LH 131I. Proc. Soc. Exp. Biol. Med. 128, 807–811 (1968)
Niswender, G.D.: Influence of the site of conjugation of the specificity of antibodies to progesterone. Steroids 22, 413 (1974)
Parlow, A.F.: In: Human Pituitary Gonadotropins (A. Albert, ed.), p. 300. Springfield (Illinois): Charles C. Thomas 1961
Pedersen, P.H., Larsen, J.F.: The ultrastructure of the human granulosa lutein cell of the first trimester of gestation. Acta. Endo. 58, 481–496 (1968)
Priedkalns, J., Weber, A.F.: Ultrastructural studies of the bovine Graafian follicle and corpus luteum. Z. Zellforsch. 91, 554–573 (1968)
Rennels, E.G.: Observations on the ultrastructure of luteal cells from PMS and PMS-HCG treated immature rats. Endocrin. 79, 373–386 (1963)
Rose, B.: Intercellular communication and some structural aspects of membrane junctions in a simple cell system. J. Mem. Biol. 5, 1–19 (1971)
Sheridan, P.J., Phillips, J.L., Simmons, D.R., Caffrey, J.L., Abel, J.H., Jr., Niswender, G.D.: Modulation of the uptake and retention of estradiol-17β in the ovine corpus luteum by luteinizing hormone. Proc. Soc. in press (1975)
Trump, B.F., Smuckler, E.A., Benditt, E.P.: A method for staining epoxy sections for light microscopy. J. Ult. Res. 5, 343–348 (1961)
Author information
Authors and Affiliations
Additional information
Supported by a grant from the Rockefeller Foundation and NIH Contract 69-2134.
Rights and permissions
About this article
Cite this article
McClellan, M.C., Diekman, M.A., Abel, J.H. et al. Luteinizing hormone, progesterone and the morphological development of normal and superovulated corpora lutea in sheep. Cell Tissue Res. 164, 291–307 (1975). https://doi.org/10.1007/BF00223011
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00223011