Abstract
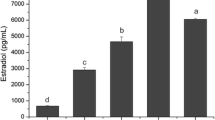
The release of epidermal growth factor ligand epiregulin (EREG) by human ovarian granulosa cells, its direct action on basic ovarian cell functions, and interrelationships with gonadotropins were investigated. We examined (1) the ovarian production of EREG (the time-dependent accumulation of EREG in the medium incubated with human ovarian granulosa cells, and (2) the effect of the addition of EREG (0, 1, 10, and 100 ng.ml−1) given alone or in combination with FSH or LH (100 ng.ml−1) on basic granulosa cells functions. Viability, proliferation (accumulation of PCNA and cyclin B1) and apoptosis (accumulation of bax and caspase 3), the release of steroid hormones (progesterone, testosterone, and estradiol), and prostaglandin E2 (PGE2) were analyzed by using the Trypan blue exclusion test, quantitative immunocytochemistry, and ELISA. A significant time-dependent accumulation of EREG in a medium cultured with human granulosa cells with a peak at 3 and 4 days was observed. The addition of EREG alone increased cell viability, proliferation, progesterone, testosterone, and estradiol release, decreased apoptosis, bud did not affect PGE2 release. The addition of either FSH or LH alone increased cell viability, proliferation, progesterone, testosterone, estradiol, and PGE2 release and decreased apoptosis. Furthermore, both FSH and LH mostly promoted the stimulatory action of EREG on granulosa cell functions. These results demonstrated, that EREG produced by ovarian cells can be an autocrine/paracrine stimulator of human ovarian cell functions. Furthermore, they demonstrate the functional interrelationship between EREG and gonadotropins in the control of ovarian functions.




Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Anderson RC, Newton CL, Anderson RA, Millar RP. Gonadotropins and their analogs: current and potential clinical applications. Endocr Rev. 2018;39(6):911–37. https://doi.org/10.1210/er.2018-00052.
Sirotkin AV. Regulators of ovarian functions. Nova Publishers Inc: New York; 2014.
Singh B, Carpenter G, Coffey RJ. EGF receptor ligands: recent advances. F1000Research. 2016;5:F1000 Faculty Rev-2270. https://doi.org/10.12688/f1000research.9025.1.
Riese DJ 2nd, Cullum RL. Epiregulin: roles in normal physiology and cancer. Semin Cell Dev Biol. 2014;28:49–56. https://doi.org/10.1016/j.semcdb.2014.03.005H.
Sekiguchi T, Mizutani T, Yamada K, Kajitani T, Yazawa T, Yoshino M, Miyamoto K. Expression of epiregulin and amphiregulin in the rat ovary. J Mol Endocrinol. 2004;33(1):281–91. https://doi.org/10.1677/jme.0.0330281.
Garnett K, Wang J, Roy SK. Spatiotemporal expression of epidermal growth factor receptor messenger RNA and protein in the hamster ovary: follicle stage-specific differential modulation by follicle-stimulating hormone, luteinizing hormone, estradiol, and progesterone. Biol Reprod. 2002;67(5):1593–604. https://doi.org/10.1095/biolreprod.102.005470.
Gall L, Chene N, Dahirel M, Ruffini S, Boulesteix C. Expression of epidermal growth factor receptor in the goat cumulus-oocyte complex. Mol Reprod Dev. 2004;67(4):439–45. https://doi.org/10.1002/mrd.20040.
Puttabyatappa M, Brogan RS, Vandevoort CA, Chaffin CL. EGF-like ligands mediate progesterone’s anti-apoptotic action on macaque granulosa cells. Biol Reprod. 2013;88(1):18. https://doi.org/10.1095/biolreprod.112.103002.
Freimann S, Ben-Ami I, Dantes A, Ron-El R, Amsterdam A. EGF-like factor epiregulin and amphiregulin expression is regulated by gonadotropins/cAMP in human ovarian follicular cells. Biochem Biophys Res Commun. 2004;324(2):829–34. https://doi.org/10.1016/j.bbrc.2004.09.129.
Qu J, Godin PA, Nisolle M, Donnez J. Distribution and epidermal growth factor receptor expression of primordial follicles in human ovarian tissue before and after cryopreservation. Hum Reprod (Oxford, England). 2000;15(2):302–10. https://doi.org/10.1093/humrep/15.2.302.
Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146(1):77–84. https://doi.org/10.1210/en.2004-0588.
Downs SM, Chen J. EGF-like peptides mediate FSH-induced maturation of cumulus cell-enclosed mouse oocytes. Mol Reprod Dev. 2008;75(1):105–14. https://doi.org/10.1002/mrd.20781.
Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science (New York, NY). 2004;303(5658):682–4. https://doi.org/10.1126/science.1092463.
Procházka R, Petlach M, Nagyová E, Nemcová L. Effect of epidermal growth factor-like peptides on pig cumulus cell expansion, oocyte maturation, and acquisition of developmental competence in vitro: comparison with gonadotropins. Reproduction (Cambridge, England). 2011;141(4):425–35. https://doi.org/10.1530/REP-10-0418.
Kim K, Lee H, Threadgill DW, Lee D. Epiregulin-dependent amphiregulin expression and ERBB2 signaling are involved in luteinizing hormone-induced paracrine signaling pathways in mouse ovary. Biochem Biophys Res Commun. 2011;405(2):319–24. https://doi.org/10.1016/j.bbrc.2011.01.039.
Sayasith K, Lussier J, Doré M, Sirois J. Human chorionic gonadotropin-dependent up-regulation of epiregulin and amphiregulin in equine and bovine follicles during the ovulatory process. Gen Comp Endocrinol. 2013;180:39–47. https://doi.org/10.1016/j.ygcen.2012.10.012.
Fang L, Cheng JC, Chang HM, Sun YP, Leung PC. EGF-like growth factors induce COX-2-derived PGE2 production through ERK1/2 in human granulosa cells. J Clin Endocrinol Metab. 2013;98(12):4932–41. https://doi.org/10.1210/jc.2013-2662.
Shimada M, Umehara T, Hoshino Y. Roles of epidermal growth factor (EGF)-like factor in the ovulation process. Reprod Med Biol. 2016;15(4):201–16. https://doi.org/10.1007/s12522-016-0236-x.
Fang L, Yu Y, Zhang R, He J, Sun YP. Amphiregulin mediates hCG-induced StAR expression and progesterone production in human granulosa cells. Sci Rep. 2016;6:24917. https://doi.org/10.1038/srep24917.
Espey LL, Richards JS. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod. 2002;67(6):1662–70. https://doi.org/10.1095/biolreprod.102.005173.
Ben-Ami I, Freimann S, Armon L, Dantes A, Ron-El R, Amsterdam A. Novel function of ovarian growth factors: combined studies by DNA microarray, biochemical and physiological approaches. Mol Hum Reprod. 2006;12(7):413–9. https://doi.org/10.1093/molehr/gal045.
Conti M, Hsieh M, Park JY, Su YQ. Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol (Baltimore, Md). 2006;20(4):715–23. https://doi.org/10.1210/me.2005-0185.
Hsieh M, Zamah AM, Conti M. Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Semin Reprod Med. 2009;27(1):52–61. https://doi.org/10.1055/s-0028-1108010.
Fabová Z, Loncová B, MlynEk M, Sirotkin AV. Interrelationships between amphiregulin, kisspeptin, FSH and FSH receptor in promotion of human ovarian cell functions. Reprod Fertil Dev. 2022;34(3):362–77. https://doi.org/10.1071/RD21230.
Sirotkin AV, Mlynček M, Kotwica J, Makarevich AV, Florkovičová I, Hetényi L. Leptin directly controls secretory activity of human ovarian granulosa cells: possible inter-relationship with the IGF/IGFBP system. Horm Res. 2005;2005(64):198–202. https://doi.org/10.1159/000089009.
Fabová Z, Sirotkin AV. Interrelationships between kisspeptin and FSH in control of porcine ovarian cell functions. Domest Anim Endocrinol. 2021;74:106520. https://doi.org/10.1016/j.domaniend.2020.106520.
Sirotkin AV, Dekanová P, Harrath AH. FSH, oxytocin and IGF-I regulate the expression of sirtuin 1 in porcine ovarian granulosa cells. Physiol Res. 2020;69(3):461–6. https://doi.org/10.33549/physiolres.934424.
Sirotkin AV, Kadasi A, Stochmalova A, Balazi A, Földesiová M, Makovicky P, Chrenek P, Harrath AH. Effect of turmeric on the viability, ovarian folliculogenesis, fecundity, ovarian hormones and response to luteinizing hormone of rabbits. Anim Int J Anim Biosci. 2018;12(6):1242–9. https://doi.org/10.1017/S175173111700235X.
Sirotkin AV, Rafay J, Kotwica J, Darlak K, Valenzuela F. Role of ghrelin in regulating rabbit ovarian function and the response to LH and IGF-I. Domest Anim Endocrinol. 2009;36(3):162–72. https://doi.org/10.1016/j.domaniend.2008.12.003.
Çelik Uzuner S. Development of a direct trypan blue exclusion method to detect cell viability of adherent cells into ELISA plates. Celal Bayar Univ J Sci. 2018;14(1):99–104. https://doi.org/10.18466/cbayarfbe.372192.
Perry SW, Epstein LG, Gelbard HA. In situ trypan blue staining of monolayer cell cultures for permanent fixation and mounting. Biotechniques. 1997;22(6):1020–4. https://doi.org/10.2144/97226bm01.
Gaumer S, Guénal I, Brun S, Théodore L, Mignotte B. Bcl-2 and Bax mammalian regulators of apoptosis are functional in Drosophila. Cell Death Differ. 2000. https://doi.org/10.1038/sj.cdd.4400714.
Osborn M, Isenberg S. Immunocytochemistry of frozen and paraffin tissue sections. In: Celis JE, editor. Cell Biology. A Laboratory Handbook, vol. 2. New York: Academic Press; 1994. p. 361–7.
Nakayama Y, Yamaguchi N. Role of cyclin B1 levelsinDNA damage and DNA damage-induced senescence. Int Rev Cell Mol Biol. 2013;305:303–37. https://doi.org/10.1016/B978-0-12-407695-2.00007-X.
Shiomi Y, Nishitani H. Control of genome integrity by RFC complexes; conductors of PCNA loading onto and unloading from chromatin during DNA replication. Genes. 2017;8(2):52. https://doi.org/10.3390/genes8020052.
An L-S, Yuan X-H, Hu Y, et al. Progesterone production requires activation of caspase-3 in preovulatory granulosa cells in a serum starvation model. Steroids. 2012;77(13):1477–82. https://doi.org/10.1016/j.steroids.2012.07.011.
Peña-Blanco A, García-Sáez AJ. Bax, Bak and beyond - mitochondrial performance in apoptosis. FEBS J. 2018;285(3):416–31. https://doi.org/10.1111/febs.14186.
Das N, Kumar TR. Molecular regulation of follicle-stimulating hormone synthesis, secretion and action. J Mol Endocrinol. 2018;60(3):R131–55. https://doi.org/10.1530/JME-17-0308.
Scaramuzzi RJ, Baird DT, Campbell BK, Driancourt MA, Dupont J, Fortune JE, Gilchrist RB, Martin GB, McNatty KP, McNeilly AS, Monget P, Monniaux D, Viñoles C, Webb R. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod Fertil Dev. 2011;23(3):444–67. https://doi.org/10.1071/RD09161.
Fru KN, Cherian-Shaw M, Puttabyatappa M, VandeVoort CA, Chaffin CL. Regulation of granulosa cell proliferation and EGF-like ligands during the periovulatory interval in monkeys. Hum Reprod (Oxford, England). 2007;22(5):1247–52. https://doi.org/10.1093/humrep/del519.
Acknowledgements
This research was financially supported by the Slovak Research and Development Agency (APVV), project APVV-15-0296, and the Scientific Grant Agency of the Ministry of Education, Science, and Sport of Slovak Republic (VEGA), project VEGA 13-ENV1321-02.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
The authors confirm the lack of potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loncová, B., Fabová, Z., Mlynček, M. et al. Assessment of Epiregulin Effect and its Combination with Gonadotropins on Proliferation, Apoptosis, and Secretory Activity by Human Ovarian Cells. Reprod. Sci. 30, 2537–2546 (2023). https://doi.org/10.1007/s43032-023-01205-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01205-z