Abstract
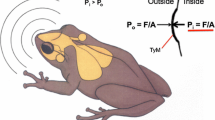
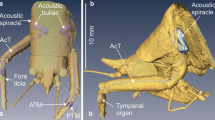
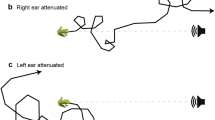
In contrast to humans and other mammals, many animals have internally coupled ears that function as inherently directional pressure-gradient receivers. Two important but unanswered questions are to what extent and how do animals with such ears exploit spatial cues in the perceptual analysis of noisy and complex acoustic scenes? This study of Cope’s gray treefrog (Hyla chrysoscelis) investigated how the inherent directionality of internally coupled ears contributes to spatial release from masking. We used laser vibrometry and signal detection theory to determine the threshold signal-to-noise ratio at which the tympanum’s response to vocalizations could be reliably detected in noise. Thresholds were determined as a function of signal location, noise location, and signal-noise separation. Vocalizations were broadcast from one of three azimuthal locations: frontal (0 °), to the right (+90 °), and to the left (−90 °). Masking noise was broadcast from each of 12 azimuthal angles around the frog (0 to 330 °, 30 ° separation). Variation in the position of the noise source resulted in, on average, 4 dB of spatial release from masking relative to co-located conditions. However, detection thresholds could be up to 9 dB lower in the “best ear for listening” compared to the other ear. The pattern and magnitude of spatial release from masking were well predicted by the tympanum’s inherent directionality. We discuss how the magnitude of masking release observed in the tympanum’s response to spatially separated signals and noise relates to that observed in previous behavioral and neurophysiological studies of frog hearing and communication.



Similar content being viewed by others
References
Bass AH, Rose GJ, Pritz MB (2005) Auditory midbrain of fish, amphibians, and reptiles: model systems for understanding auditory function. In: The inferior colliculus, pp 459–492: Springer.
Bee MA (2007) Sound source segregation in grey treefrogs: spatial release from masking by the sound of a chorus. Anim Behav 74:549–558
Bee MA (2008) Finding a mate at a cocktail party: spatial release from masking improves acoustic mate recognition in grey treefrogs. Anim Behav 75:1781–1791
Bee MA (2012) Sound source perception in anuran amphibians. Curr Opin Neurobiol 22:301–310
Bee MA (2015) Treefrogs as animal models for research on auditory scene analysis and the cocktail party problem. Int J Psychophysiol 95:216–237
Bee MA, Micheyl C (2008) The cocktail party problem: what is it? How can it be solved? And why should animal behaviorists study it? J Comp Psychol 122:235–251
Bee MA, Schwartz JJ (2009) Behavioral measures of signal recognition thresholds in frogs in the presence and absence of chorus-shaped noise. J Acoust Soc Am 126:2788–2801
Bronkhorst AW (2000) The cocktail party phenomenon: a review of research on speech intelligibility in multiple-talker conditions. Acustica 86:117–128
Bronkhorst AW, Plomp R (1988) The effect of head-induced interaural time and level differences on speech intelligibility in noise. J Acoust Soc Am 83:1508–1516
Brumm H (ed) (2013) Animal communication and noise. Springer, New York
Buerkle NP, Schrode KM, Bee MA (2014) Assessing stimulus and subject influences on auditory evoked potentials and their relation to peripheral physiology in green treefrogs (Hyla cinerea). Comp Biochem Physiol A 178:68–81
Caird D, Pillmann F, Klinke R (1989) Responses of single cells in the cat inferior colliculus to binaural masking level difference signals. Hear Res 43:1–23
Caldwell MS, Bee MA (2014) Spatial hearing in Cope's gray treefrog: I. Open and closed loop experiments on sound localization in the presence and absence of noise. Journal of Comparative Physiology A 200:265–284
Caldwell MS, Lee N, Schrode KM, Johns AR, Christensen-Dalsgaard J, Bee MA (2014) Spatial hearing in Cope's gray treefrog: II. Frequency-dependent directionality in the amplitude and phase of tympanum vibrations. Journal of Comparative Physiology A 200:285–304
Christensen-Dalsgaard J (2005) Directional hearing in nonmammalian tetrapods. In: Popper AN, Fay RR (eds) Sound source localization. Springer, New York, pp 67–123
Christensen-Dalsgaard J (2011) Vertebrate pressure-gradient receivers. Hear Res 273:37–45
Dent ML, McClaine EM, Best V, Ozmeral E, Narayan R, Gallun FJ, Sen K, Shinn-Cunningham BG (2009) Spatial unmasking of birdsong in zebra finches (Taeniopygia guttata) and budgerigars (Melopsittacus undulatus). J Comp Psychol 123:357–367
Ehret G, Tautz J, Schmitz B, Narins PM (1990) Hearing through the lungs: lung-eardrum transmission of sound in the frog Eleutherodactylus coqui. Naturwissenschaften 77:192–194
Feng AS, Gerhardt HC, Capranica RR (1976) Sound localization behavior of the green treefrog (Hyla cinerea) and the barking treefrog (Hyla gratiosa). J Comp Physiol 107:241–252
Gerhardt HC (2001) Acoustic communication in two groups of closely related treefrogs. Adv Study Behav 30:99–167
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago University Press, Chicago
Green DM, Swets JA (1966) Signal detection theory and psychophysics. John Wiley & Sons, New York
Greenhouse SW, Geisser S (1959) On methods in the analysis of profile data. Psychometrika 24:95–112
Hine JE, Martin RL, Moore DR (1994) Free-field binaural unmasking in ferrets. Behav Neurosci 108:196–205
Ho CCK, Narins PM (2006) Directionality of the pressure-difference receiver ears in the northern leopard frog, Rana pipiens pipiens. J Comp Physiol A 192:417–429
Holt MM, Schusterman RJ (2007) Spatial release from masking of aerial tones in pinnipeds. J Acoust Soc Am 121:1219–1225
Ison JR, Agrawal P (1998) The effect of spatial separation of signal and noise on masking in the free field as a function of signal frequency and age in the mouse. J Acoust Soc Am 104:1689–1695
Jørgensen MB (1991) Comparative studies of the biophysics of directional hearing in anurans. J Comp Physiol A 169:591–598
Jørgensen MB, Gerhardt HC (1991) Directional hearing in the gray tree frog Hyla versicolor: eardrum vibrations and phonotaxis. J Comp Physiol A 169:177–183
Jørgensen MB, Schmitz B, Christensen-Dalsgaard J (1991) Biophysics of directional hearing in the frog Eleutherodactylus coqui. J Comp Physiol A 168:223–232
Lin WY, Feng AS (2001) Free-field unmasking response characteristics of frog auditory nerve fibers: comparison with the responses of midbrain auditory neurons. J Comp Physiol A 187:699–712
Lin WY, Feng AS (2003) GABA is involved in spatial unmasking in the frog auditory midbrain. J Neurosci 23:8143–8151
McDermott JH (2009) The cocktail party problem. Curr Biol 19:R1024–R1027
Michelsen A, Larsen ON (2008) Pressure difference receiving ears. Bioinspir Biomim 3:011001
Narins PM (1995) Frog communication. Sci Am 273:78–83
Nityananda V, Bee MA (2011) Finding your mate at a cocktail party: frequency separation promotes auditory stream segregation of concurrent voices in multi-species frog choruses. PLoS One 6, e21191
Nityananda V, Bee MA (2012) Spatial release from masking in a free-field source identification task by gray treefrogs. Hear Res 285:86–97
Ptacek MB, Gerhardt HC, Sage RD (1994) Speciation by polyploidy in treefrogs: multiple origins of the tetraploid, Hyla versicolor. Evolution 48:898–908
Ratnam R, Feng AS (1998) Detection of auditory signals by frog inferior collicular neurons in the presence of spatially separated noise. J Neurophysiol 80:2848–2859
Rheinlaender J, Gerhardt HC, Yager DD, Capranica RR (1979) Accuracy of phonotaxis by the green treefrog (Hyla cinerea). J Comp Physiol 133:247–255
Richardson C, Lengagne T (2010) Multiple signals and male spacing affect female preference at cocktail parties in treefrogs. Proc R Soc B-Biol Sci 277:1247–1252
Robert D (2005) Directional hearing in insects. In: Popper AN, Fay RR (eds) Sound source localization. Springer, New York, NY, pp 6–35
Römer H (2013) Masking by noise in acoustic insects: problems and solutions. In: Brumm H (ed) Animal communication and noise. Springer, New York, pp 33–63
Römer H (2015) Directional hearing: from biophysical binaural cues to directional hearing outdoors. J Comp Physiol A 201:87–97
Rose GJ, Gooler DM (2007) Function of the amphibian central auditory system. In: Narins PA, Feng AS, Fay RR, Popper AN (eds) Hearing and sound communication in amphibians. Springer, New York, pp 250–290
Schmidt AKD, Römer H (2011) Solutions to the cocktail party problem in insects: selective filters, spatial release from masking and gain control in tropical crickets. PLoS One 6, e28593
Schwartz JJ, Gerhardt HC (1989) Spatially mediated release from auditory masking in an anuran amphibian. J Comp Physiol A 166:37–41
Simpson AJ, Fitter MJ (1973) What is the best index of detectability? Psychol Bull 80:481–488
Vélez A, Schwartz JJ, Bee MA (2013) Anuran acoustic signal perception in noisy environments. In: Brumm H (ed) Animal communication and noise. Springer, New York, pp 133–185
Ward JL, Buerkle NP, Bee MA (2013a) Spatial release from masking improves sound pattern discrimination along a biologically relevant pulse-rate continuum in gray treefrogs. Hear Res 306:63–75
Ward JL, Love EK, Vélez A, Buerkle NP, O'Bryan LR, Bee MA (2013b) Multitasking males and multiplicative females: dynamic signalling and receiver preferences in Cope's grey treefrog. Anim Behav 86:231–243
Warnecke M, Bates ME, Flores V, Simmons JA (2014) Spatial release from simultaneous echo masking in bat sonar. J Acoust Soc Am 135:3077–3085
Wilczynski W, Ryan MJ (2010) The behavioral neuroscience of anuran social signal processing. Curr Opin Neurobiol 20:754–763
Wiley RH (2015) Noise matters: the evolution of communication. Harvard University Press, Cambridge, MA
Zurek PM (1992) Binaural advantages and directional effects in speech intelligibility. In: Studebaker GA, Hochberg I (eds) Acoustical factors affecting hearing aid performance. Allyn and Bacon, Boston, pp 255–276
Acknowledgments
We thank Katrina Schrode for providing code used in data analysis, Jessica Ward for logistical support, Shelby Seckora, Nathan Buerkle, and Sam Levin for assistance in data collection, and three anonymous reviewers for helpful feedback on earlier drafts. This research was supported by a grant from the National Institute on Deafness and Other Communication Disorders (R01 DC009582).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Caldwell, M.S., Lee, N. & Bee, M.A. Inherent Directionality Determines Spatial Release from Masking at the Tympanum in a Vertebrate with Internally Coupled Ears. JARO 17, 259–270 (2016). https://doi.org/10.1007/s10162-016-0568-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-016-0568-6