Abstract
The electrosensory lobe of mormyrid fish Gnathonemus petersii is strongly affected by corollary discharge signals associated with the electric organ discharge (EOD) motor command. This study is a physiological examination of one important source of corollary discharge signals to the lobe, the medial juxtalobar nucleus.
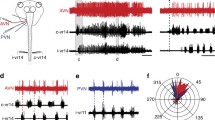
Intracellular recordings from neurons of the medial juxtalobar nucleus show a single corollary discharge driven spike occurring at about 7.5 ms after the initiation of the EOD motor command with a temporal jitter in individual cells of less than 0.05 ms. Responses to intracellular current injection suggest the presence of a low threshold outward current that is similar to outward currents that have been identified in those neurons of the auditory system in which precise temporal information is preserved.
Results from stimulation and lesion experiments indicate that the medial juxtalobar nucleus is responsible for major corollary discharge effects in the mormyromast regions of the electrosensory lobe, including: 1) the gate-like excitation of granule cells; and 2) various excitatory effects on other cells of the lobe. The medial juxtalobar nucleus transmits precise temporal information about the timing of the EOD motor command to the electrosensory lobe. This information is probably used in decoding electrosensory afferent latency as a measure of stimulus intensity.
Similar content being viewed by others
Abbreviations
- Ad :
-
antidromic
- bc :
-
bulbocerebellar tract
- BCA :
-
bulbar command associated nucleus
- CD :
-
corollary discharge
- EGp :
-
eminentia granularis posterior
- ELL :
-
electrosensory lobe
- ELLga :
-
electrosensory lobe, ganglion layer
- ELLgr :
-
electrosensory lobe, granule layer
- ELLml :
-
molecular layer of ELL cortex
- EOD :
-
electric organ discharge
- JLl :
-
lateral juxtalobar nucleus
- JLm :
-
medial juxtalobar nucleus
- MCA :
-
mesencephalic command associated nucleus
- MO :
-
medial octavolateral nucleus
- MOml :
-
molecular layer of the medial octavolateral nucleus
- nALL :
-
anterior lateral line nerve
- PCA :
-
paratrigeminal command associated nucleus
References
Baker R, Llinas R (1971) Electrotonic coupling between neurons in the rat mesencephalic nucleus. J Physiol (Lond) 212: 45–63
Bell CC, Grant K (1989) Corollary discharge inhibition and preservation of temporal information in a sensory nucleus of mormyrid electric fish. J Neurosci 9: 1029–1044
Bell CC (1990a) Mormyromast electroreceptor organs and their afferents in mormyrid electric fish: II. Intra-axonal recordings show initial stages of central processing. J Neurophysiol 63: 303–318
Bell CC (1990b) Mormyromast electroreceptor organs and their afferents in mormyrid electric fish. III. Physiological differences between two morphological types of fibers. J Neurophysiol 63: 319–332
Bell CC, Finger TE, Russell CJ (1981) Central connections of the posterior lateral line lobe in mormyrid fish. Exp Brain Res 42: 9–22
Bell CC, Libouban S, Szabo T (1983) Pathways of the electric organ discharge command and its corollary discharges in mormyrid fish. J Comp Neurol 216: 327–338
Bell CC, Grant K, Serrier J (1992) Corollary discharge effects and sensory processing in the mormyrid electrosensory lobe. I. Field potentials and cellular activity in associated structures. J Neurophysiol 68: 843–858
Bell CC, Dunn K, Hall C, Caputi A (1995) Electric organ discharge pathways in mormyrid fish. I. The mesencephalic command associated nucleus. J Comp Physiol A 177: 449–462
Bell CC (1989) Sensory coding and corollary discharge effects in mormyrid electric fish. J Exp Biol 146: 229–253
Bennett MVL (1977) Electrical transmission: a functional analysis and comparison to chemical transmission. In: Brookhart JM et al. (eds) Handbook of physiology. Section I, vol. I. Cellular biology of neurons, Part I. Am Physiol Soc, Bethesda, pp 357–416
Carr CE (1986) Time coding in electric fish and barn owls. Brain Behav Evol 28: 122–133
Carr CE, Maler L, Taylor B (1986) A time-comparison circuit in the electric fish midbrain. II. Functional morphology. J Neurosci 6: 1372–1383
Grant K, Bell CC, Clausse S, Ravaille M (1986) Morphology and physiology of the brainstem nuclei controlling the electric organ discharge in mormyrid fish. J Comp Neurol 245: 514–530
Gustafsson B, Jankowska E (1976) Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol (Lond) 258: 33–61
Hall R, Bell C, Zelick R (1995) Behavioral evidence of a latency code for stimulus intensity in mormyrid electric fish. J Comp Physiol A 177: 29–39
Hille B (1984) Ionic channels of excitable membranes. Sinauer Assoc., Sunderland, Mass
Jankowska E, Padel Y, Tanaka R (1975) The mode of activation of pyramidal tract cells by intracortical stimuli. J Physiol (Lond) 249: 617–636
Jhaveri SR, Morest DK (1982) Neuronal architecture in the nucleus magnocellularis of the chicken auditory system with observations on nucleus laminaris: a light and electron microscope study. Neuroscience 7: 809–835
Konishi M, Takahashi TT, Wagner W, Sullivan WE, Carr CE (1988) Neurophysiological and anatomical substrates of sound localization in the owl. In: Edelman GM, Gall WE, Cowan WM (eds) Auditory function: neurobiological basis of hearing. John Wiley and Sons, New York, pp 721–745
Llinás R, Baker R, Sotelo C (1974) Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol 37: 560–571
Losier BJ, Matsubara JA (1990) Light and electron microscopical studies on the spherical neurons in the electrosensory lateral line lobe of the gymnotiform fish, Sternopygus. J Comp Neurol 298: 237–249
Maler L, Sas EKB, Rogers J (1981) The cytology of the posterior lateral line lobe of high frequency weakly electric fish (Gymnotidae): dendritic differentiation and synaptic specificity in a simple cortex. J Comp Neurol 195: 87–140
Manis PB, Marx SO (1991) Outward currents in isolated ventral cochlear nucleus neurons. J Neurosci 11: 2865–2880
Mugnaini E, Maler L (1987) Cytology and immunohistochemistry of the nucleus exterolateralis anterior of the mormyrid brain: possible role of GABAergic synapses in temporal analysis. Anat Embryol 176: 313–336
Reyes AD, Rubel EW, Spain WJ (1994) Membrane properties underlying the firing of neurons in the avian cochlear nucleus. J Neurosci 14: 5352–5364
Smith PH, Rhode WS (1987) Characterization of HRP labeled globular bushy cells in the cat anteroventral cochlear nucleus. J Comp Neurol 266: 360–375
Stuart G, Hausser M (1994) Initiation and spread of sodium action potentials in cerebellar Purkinje cells. Neuron 13: 703–712
Szabo T, Hagiwara S (1967) A latency-change mechanism involved in sensory coding of electric fish (mormyrids). Physiol Behav 2: 331–335
Szabo T, Ravaille M, Libouban S, Enger DS (1983) The mormyrid rhombencephalon. I. Light and EM investigations on the structure and connections of the lateral line lobe nucleus with HRP labeling. Brain Res 266: 1–19
Wu SH, Oertel D (1984) Intracellular injection with horseradish peroxidase of physiologically characterized stellate and bushy cells in slices of mouse anteroventral cochlear nucleus. J Neurosci 4: 1577–1588
Zhang S, Trussell LO (1994) A characterization of excitatory postsynaptic potentials in the avian nucleus magnocellularis. J Neurophysiol: 72 705–718
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bell, C., von der Emde, G. Electric organ corollary discharge pathways in mormyrid fish. J Comp Physiol A 177, 463–479 (1995). https://doi.org/10.1007/BF00187482
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00187482