Abstract
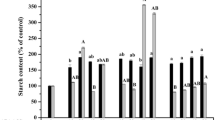
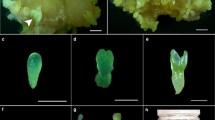
Different carbohydrates were investigated for somatic embryo development of black spruce and red spruce. They were tested in a basal maturation medium consisting of Litvay's salts at half-strength containing 1 g l-1 glutamine, 1 g l-1 casein hydrolysate, 7.5 μM abscisic acid, and 0.9% Difco Bacto-agar. A comparison of different sucrose concentrations showed that 6% was optimal for embryo development. Among the nine carbohydrates tested, sucrose, fructose, glucose, maltose, and cellobiose supported embryo development while arabinose, mannitol, myo-inositol, and sorbitol did not. A comparison of sucrose, glucose, and fructose at three concentrations showed that the general pattern of response for both species followed concentration expressed as a percentage, independent of the molarity of carbohydrate in the medium. Interspecific differences were observed concerning carbohydrate requirements. For red spruce, 6% fructose was found best for embryo development, while no such preference was observed for black spruce. No significant difference was observed in the number of embryos produced with 6% sucrose or 3% sucrose plus an equimolar concentration of either mannitol, sorbitol, or myo-inositol in the maturation medium, suggesting that the effect of the carbohydrate on the maturation was partly osmotic.
Similar content being viewed by others
References
Batty N & Dunwell J (1989) Effect of maltose on the response of potato anthers in culture. Plant Cell Tiss. Org. Cult. 18: 221–226
Becwar MR, Noland NL & Wyckoff JL (1989) Maturation, germination, and conversion of Norway spruce (Picea abies L.) somatic embryos to plants. In Vitro Cell. Dev. Biol. 25: 575–580
Bercetche J (1988) Optimisation des conditions d'obtention de plantules à partir de cals embryogènes chez Picea abies. Ann. AFOCEL 97–115
Boulay MP, Gupta PK, Krogstrup P & Durzan DJ (1988) Development of somatic embryos from cell suspension cultures of Norway spruce (Picea abies Karst.). Plant Cell Rep. 7: 134–137
Campbell RA & Durzan DJ (1975) Induction of multiple buds and needles in tissue cultures of Picea glauca. Can. J. Bot. 53: 1652–1657
Dunstan DI (1988) Prospects and progress in conifer biotechnology. Can. J. For. Res. 18: 1497–1506
Dunstan DI, Bekkaoui F, Pilon M, Fowke LC & Abrams SR (1988) Effects of abscisic acid and analogues on the maturation of white spruce (Picea glauca) somatic embryos. Plant Sci. 58: 77–84
Durzan DJ & Gupta PK (1987) Somatic embryogenesis and polyembryogenesis in Douglas-fir cell suspension cultures. Plant Sci. 52: 229–235
Finer JJ, Kriebel HB & Becwar MR (1989) Initiation of embryogenic callus and suspension cultures of eastern white pine (Pinus strobus L.). Plant Cell Rep. 8: 203–206
Gupta PK & Durzan DJ (1986) Plantlet regeneration via somatic embryogenesis from subcultured callus of mature embryos of Picea abies (Norway spruce). In Vitro Cell. Dev. Biol. 22: 685–688
Hakman I & vonArnold S (1988) Somatic embryogenesis and plant regeneration from suspension cultures of Picea glauca (white spruce). Physiol. Plant. 72: 579–587
Jain SM, Newton RJ & Soltes EJ (1988) Enhancement of somatic embryogenesis in Norway spruce (Picea abies L.). Theor. Appl. Genet. 76: 501–506
Kononowicz AK & Janick J (1984) The influence of carbon source on the growth and development of asexual embryos of Theobroma cacao. Physiol. Plant. 61: 155–162
Litvay JD, Verma DC & Johnson MA (1985) Influence of loblolly pine (Pinus taeda L.). Culture medium and its components on growth and somatic embryogenesis of the wild carrot (Daucus carota L.). Plant Cell Rep. 4: 325–328
La C-Y & Thorpe TA (1987) Somatic embryogenesis and plantlet regeneration in cultured immature embryos of Picea glauca. J. Plant Physiol. 128: 297–302
Nash DT & Boll WG (1975) Carbohydrate nutrition of Paul's Scarlet rose cell suspensions. Can. J. Bot. 53: 179–185
Roberts DR, Flinn BS, Webb DT, Webster FB & Sutton BCS (1990) Abscisic acid and indole-3-butyric acid regulation of maturation and accumulation of storage proteins in somatic embryos of interior spruce. Physiol. Plant. 78: 355–360
Schenk RU & Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 50: 199–204
Sorvari S & Schieder O (1987) Influence of sucrose and melbiose on barley anther cultures in starch media. Plant Breed. 99: 164–171
Steel RGD & Torrie JH (1980) Principles and Procedures of Statistics: A Biometrical Approach. 2nd edition, McGraw-Hill Book Company, 633 p
Strickland SG, Nichol JW, McCall CM & Stuart DA (1987) Effect of carbohydrate source on alfalfa somatic embryogenesis. Plant Sci. 48: 113–121
Thompson MR, Thorpe TA (1987) Metabolic and non metabolic roles of carbohydrates. In: Bonga JM & Durzan J (Eds) Cell and Tissue Culture in Forestry, Vol 1 (pp 89–112) Martinus Nijhoff Publishers, Dordrecht
Tremblay FM (1990) Somatic embryogenesis and plantlet regeneration from embryos isolated from stored seeds of Picea glauca. Can. J. Bot. 68: 236–242
Tremblay L & Tremblay FM. Effects of gelling agents, ammonium nitrate, and light on the development of Picea mariana (Mill) B.S.P. (black spruce) and P. rubens Sarg. (red spruce) somatic embryos. Plant Sci. (in press)
Verma DC & Dougall DK (1977) Influence of carbohydrates on quantitative aspects of growth and embryo formation in wild carrot suspension cultures. Plant Physiol. 59: 81–85
vonArnold S & Hakman I (1988) Regulation of somatic embryo development in Picea abies by abscisic acid (ABA). J. Plant Physiol. 132: 164–169
Vuke TM & Mott RL (1987) Growth of loblolly pine callus on a variety of carbohydrate sources. Plant Cell Rep. 6: 153–156
Webster FB, Roberts DR, McInnis SM & Sutton BCS (1990) Propagation of interior spruce by somatic embryogenesis. Can. J. For. Res. 20: 1759–1765
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tremblay, L., Tremblay, F.M. Carbohydrate requirements for the development of black spruce (Picea mariana (Mill.) B.S.P.) and red spruce (P. rubens Sarg.) somatic embryos. Plant Cell Tiss Organ Cult 27, 95–103 (1991). https://doi.org/10.1007/BF00048213
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00048213