Summary
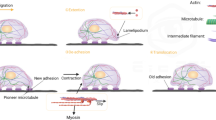
The machinery for cell locomotion is based in a network of polymerized actin filaments supporting the peripheral cytoplasm. This network or ‘gel’ consists of actin filaments in a variety of configurations, including cables, loose bundles, and branching arrays; all formed by the interaction of actin-associated proteins with actin filaments. For cell locomotion to occur, this network must be reversibly disassembled or ‘solated” to allow protrusion, then re-assembled to stabilize the resulting extension. Thus, proteins to promote both ‘solation’ and ‘gelation’ of actin are important for efficient cell locomotion. Because of their distribution, control, and in vitro effects on actin filaments, two such proteins, gelsolin and actin-binding protein (ABP) should play especially important roles in cell motility. Support for this premise is found in in vivo studies of mouse kidney fibroblasts which demonstrated increased translocational locomotion after cytoplasmic gelsolin expression was increased genetically and in melanoma cells missing actin-binding protein which behave as expected for a cell unable to achieve efficient actin gelation. Since malignant transformation is known to affect the expression and distribution of several of these actin structural proteins, including gelsolin, further investigations of the role these proteins play in cell motility will be important to the determination of tumor cell motility and hence metastatic propensity.
Similar content being viewed by others
References
Muller OF: Animalcula infusoria fluviatilia et marina, quae detexit systematice descipset et ad vivum delineari curavit. In: Molleri N, Copenhagen (ed) 10–11, 1786
Stossel TP, Janmey PA, Zaner KS: Cytomechanics. In: Bereiter-Han J, Anderson OR, Reif WE (ed) Cytomechanics. Springer, Berlin 131–153, 1987
Mooseker MS: Annu Rev Cell Biol 1: 209–241, 1985
Burridge K, Fath K, Kelly T, Nuckolls G, Turner C: Annu Rev Cell Biol 4: 487–525, 1988
Hartwig JH, Shevlin P: The architecture of actin filaments and the ultrastructural location of actin-binding protein in the periphery of lung macrophages. J Cell Biol 103: 1007–20, 1986
Stossel TP: Contribution of actin to the structure of the cytoplasmic matrix. J Cell Biol 99: 15s-21s, 1984
Schleicher M, Andre E, Hartmann H, Noegel AA: Actin-binding proteins are conserved from slime molds to man. Dev Genet 9: 521–30, 1988
Meyer RK, Aebi U: Bundling of actin filaments by alpha-actinin depends on its molecular length. J Cell Biol 110: 2013–24, 1990
Koenig M, Monaco AP, Kunkel LM: The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 53: 219–26, 1988
Noegel AA, Rapp S, Lottspeich F, Schleicher M, Stewart M: The Dictyostelium gelation factor shares a putative actin binding site with alpha-actinins and dystrophin and also has a rod domain containing six 100-residue motifs that appear to have a cross-beta conformation. J Cell Biol 109: 607–18, 1989
Gorlin Y, Yamin R, Egan S, Stewart M, Stossel T, Kwiatkowski D, Hartwig J: Human Endothelial Actin-binding Protein (ABP-280, Nonmuscle Filamin); A Molecular Leaf Spring. JCB 111: 1089–1105, 1990
Byers T, Husain-Chishti A, Dubreil R, Branton D, Goldstein L: Sequence similarity of the amino-terminal domain of Drosophilia beta spectrin to alpha-actinin and dystrophin. J Cell Biol 109: 1633–1641, 1989
de Arruda M, Watson S, Lin C-S, Leavitt J, Matsudaira P: Fimbrin is a homologue of the cytoplasmic phosphoprotein plastin and has domains homologous with calmodulin and actin gelation proteins. J Cell Biol 111: 1069–1079, 1990
Hartwig JH, Desisto M: The Cytoskeleton of the Resting Human Blood Platelet: Structure of the Membrane Skeleton and Its Attachment to Actin Filaments. JCB 112: 407–425, 1991
Brotschi EA, Hartwig JH, Stossed TP: The gelation of actin by actin-binding protein. J Biol Chem 253: 8988–93, 1978
Mimura N, Asuro A: Actin-related gelation of Ehrlich tumour cell extracts is reversibly inhibited by low concentrations of Ca2+. Nature 272: 273–276, 1978
Corwin HL, Hartwig JH: Isolation of actin-binding protein and villin from toad oocytes. Dev Biol 99: 61–74, 1983
Hartwig JH, Stossel TP: Isolation and properties of actin, myosin, and a new actinbinding protein in rabbit alveolar macrophages. J Biol Chem 250: 5696–705, 1975
Wang K, Ash JF, Singer SJ: Filamin, a new high-molecular-weight protein found in smooth muscle and non-muscle cells. Proc Natl Acad Sci USA 72: 4483–6, 1975
Hock RS, Davis G, Speicher DW: Purification of human smooth muscle filamin and characterization of structural domains and functional sites. Biochemistry 29: 9441–51, 1990
Janmey PA, Hvidt S, Lamb J, Stossel TP: Resemblance of actin-binding protein/actin gels to covalently crosslinked networks. Nature 345: 89–92, 1990
Cunningham CC, Byers HR, Hartwig JH, Stossel TP, Kwiatkowski DJ: Morphologic Properties of Three Actin-binding Protein (ABP, non-muscle filamin)-Deficient Human Malignant Melanoma Cell Lines are Consistent with Reduced Cortical Actin Gelation. J Cell Biol 109: 277a, 1989
Davies WA, Stossel TP: Peripheral hyaline blebs (podosomes) of macrophages. J Cell Biol 75: 941–55, 1977
Mesland DA, Los G, Spiele H: Cytochalasin B disrupts the association of filamentous web and plasma membrane in hepatocytes. Exp Cell Res 135: 431–5, 1981
Weiss E, Sterz I, Frimmer M, Kroker R: Electron microscopy of isolated rat hepatocytes before and after treatment with phalloidin. Beitr Pathol 150: 345–56, 1973
Fox JE: Identification of actin-binding protein as the protein linking the membrane skeleton to glycoproteins on platelet plasma membranes. J Biol Chem 260: 11970–7, 1985
Okita JR, Pidard D, Newman PJ, Montgomery RR, Kunicki TJ: On the association of glycoprotein Ib and actin-binding protein in human platelets. J Cell Biol 100: 317–21, 1985
Ezzell RM, Kenney DM, Egan S, Stossel TP, Hartwig JH: Localization of the domain of actin-binding protein that binds to membrane glycoprotein Ib and actin in human platelets. J Biol Chem 263: 13303–9, 1988
Ohta Y, Stossel TP, Hartwig JH: Ligand-Sensitive Binding of Actin-binding Protein (ABP) to Immunoglobulin G Fc Receptor I (FcγRI, CD64). Cell (in press): 1991
Cunningham CC, Gorlin JB, Kwiatkowski DJ, Hartwig JH, Janmey PJ, Byers HR, Stossel TP: Genetic Repair of Cortically Unstable Human Melanoma Cells Deficient in Actin-binding Protein. Science (in press): 1991
Andre E, Lottspeich F, Schleicher M, Noegel A: Severin, gelsolin, and villin share a homologous sequence in regions presumed to contain F-actin severing domains. J Biol Chem 263: 722–7, 1988
Janmey PA, Stossel TP: Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature 253: 362–4, 1987
Janmey PA, Matsudaira PT: Functional comparison of villin and gelsolin. Effects of Ca2+, KCl, and polyphosphoinositides. J Biol Chem 263: 16738–43, 1988
Yin HL, Janmey PA, Schleicher M: Severin is a gelsolin prototype. Febs Lett 264: 78–80, 1990
Bretscher A, Weber K: Villin is a major protein of microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell 20: 839–847, 1980
Sakurai T, Kurokawa H, Nonomura Y: The Ca2+-dependent Actin Filament-severing Activity of 74-kDa Protein (Adseverin) Resides in NTs NH2-terminal Half. J Biol Chem 266: 4581–4585, 1991
Rodriquez DCA, Lemaire S, Tchakarov L, Jeyapragasan M, Doucet JP, Vitale ML, Trifaro JM: Chromaffin cell scinden a novel calcium-dependent actin filament-severing protein. Embo J 9: 43–52, 1990
Cunningham CC, Stossel TP, Kwiatkowski DJ: Enhanced Motility in NIH 3T3 Fibroblasts That Overexpress Gelsolin. Science 251: 1233–1236, 1991
Leavitt J, Gunning P, Kedes L, Jariwalla R: Smooth muscle alpha-actin is a transformation-sensitive marker for mouse NIH 3T3 and Rat-2 cells. Nature 316: 840–2, 1985
Takahashi K, Heine UI, Junker JL, Colburn NH, Rice JM: Role of cytoskeleton changes and expression of the H-ras oncogene during promotion of neoplastic transformation in mouse epidermal JB6 cells. Cancer Res 46: 5923–32, 1986
Koffer A, Daridan M, Clarke G: Regulation of the Microfilament System in Normal and Polyoma Virus Transformed Cultured (BHK) Cells. Tissue & Cell 17: 147–159, 1985
Kakunaga T: Malignant transformation and alteration of actin-related proteins. Gan To Kagaku Ryoho 1987
Wang E, Goldberg AR: Changes in microfilament organization and surface topography upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc Natl Acad Sci USA 73: 4065–9, 1976
Fujita H, Suzuki H, Kuzumaki N, Mullauer L, Ogiso Y, Oda A, Ebisawa K, Sakurai T, Nonomura Y, Kijimoto OS: A specific protein, p92, detected in flat revertants derived from NIH/3T3 transformed by human activated c-Ha-ras oncogene. Exp Cell Res 186: 115–21, 1990
Vandekerckhove J, Bauw G, Vancompernolle K, Honore B, Celis J: Comparative two-dimensional gel analysis and microsequencing identifies gelsolin as one of the most prominent downregulated markers of transformed human fibroblast and epithelial cells. J Cell Biol 111: 95–102, 1990
Banyard MR, Medveczyk CJ, Tellam RL: Microfilament organization correlates with increased cellular content of gelsolin. Exp Cell Res. 199, Mar 1990
Chaponnier C, Gabbiani G; Gelsolin modulation in epithelial and stromal cells of mammary carcinoma. Am J Pathol 134: 597–603, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Casey Cunningham, C. Actin structural proteins in cell motility. Cancer Metast Rev 11, 69–77 (1992). https://doi.org/10.1007/BF00047604
Issue Date:
DOI: https://doi.org/10.1007/BF00047604