Abstract
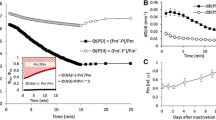
Photoinhibition of photosynthesis was studied in intact barley leaves at 5 and 20°C, to reveal if Photosystem II becomes predisposed to photoinhibition at low temperature by 1) creation of excessive excitation of Photosystem II or, 2) inhibition of the repair process of Photosystem II. The light and temperature dependence of the reduction state of QA was measured by modulated fluorescence. Photon flux densities giving 60% of QA in a reduced state at steady-state photosynthesis (300 μmol m−2s−1 at 5°C and 1200 μmol m−2s−1 at 20°C) resulted in a depression of the photochemical efficiency of Photosystem II (Fv/Fm) at both 5 and 20°C. Inhibition of Fv/Fm occurred with initially similar kinetics at the two temperatures. After 6h, Fv/Fm was inhibited by 30% and had reached steady-state at 20°C. However, at 5°C, Fv/Fm continued to decrease and after 10h, Fv/Fm was depressed to 55% of control. The light response of the reduction state of QA did not change during photoinhibition at 20°C, whereas after photoinhibition at 5°C, the proportion of closed reaction centres at a given photon flux density was 10–20% lower than before photoinhibition.
Changes in the D1-content were measured by immunoblotting and by the atrazine binding capacity during photoinhibition at high and low temperatures, with and without the addition of chloramphenicol to block chloroplast encoded protein synthesis. At 20°C, there was a close correlation between the amount of D1-protein and the photochemical efficiency of photosystem II, both in the presence or in the absence of an active repair cycle. At 5°C, an accumulation of inactive reaction centres occurred, since the photochemical efficiency of Photosystem II was much more depressed than the loss of D1-protein. Furthermore, at 5°C the repair cycle was largely inhibited as concluded from the finding that blockage of chloroplast encoded protein synthesis did not enhance the susceptibility to photoinhibition at 5°C.
It is concluded that, the kinetics of the initial decrease of Fv/Fm was determined by the reduction state of the primary electron acceptor QA, at both temperatures. However, the further suppression of Fv/Fm at 5°C after several hours of photoinhibition implies that the inhibited repair cycle started to have an effect in determining the photochemical efficiency of Photosystem II.
Similar content being viewed by others
Abbreviations
- CAP:
-
D-threochloramphenicol
- F0 and F′ 0 :
-
fluorescence when all Photosystem II reaction centres are open in dark- and light-acclimated leaves, respectively
- Fm and F′ m :
-
fluorescence when all Photosystem II reaction centres are closed in dark- and light-acclimated leaves, respectively
- Fs :
-
fluorescence at steady state
- QA :
-
the primary, stable quinone acceptor of Photosystem II
- qN :
-
non-photochemical quenching of fluorescence
- qP :
-
photochemical quenching of fluorescence
References
Andersson B, Salter HA, Virgin I, Vass I and Styring S (1992) Photodamage to Photosystem II—primary and secondary events. J Photochem Photobiol Biol 15: 15–31
Arnon DI (1948) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24: 1–15
Aro E-M, Hundal T, Carlberg I and Andersson B (1990) In vitro studies on light-induced inhibition of Photosystem II and D1-protein degradation at low temperatures. Biochim Biophys Acta 1019: 269–275
Bilger W and Björkman O (1991) Temperature dependence of violaxanthin de-epoxidation and non-photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parviflora L. Planta 184: 226–234
Björkman O (1987) Low-temperature chlorophyll fluorescence in leaves and its relation to photon yield of photosynthesis in photoinhibition. In: Kyle DJ, Osmond CB and Arntzen CJ (eds) Topics in Photosythesis, Vol 9, pp123–144. Elsevier, Amsterdam
Chow WS, Osmond CB and Huang LK (1989) Photosystem II function and herbicide binding sites during photoinhibition of chloroplasts in-vivo and in-vitro. Photosynth Res 21: 17–26
Demmig-Adams B, Adams WW, Heber U, Neimanis S, Winter K, Krüger A, Czygan F-C, Bilger W and Björkman O (1990) Inhibition of zeaxanthin formation and of rapid changes in radiationless energy dissipation by dithiothreitol in spinach leaves and chloroplasts. Plant Physiol 92: 293–301
Demming B and Björkman O (1987) Comparison of the effect of excessive light on chlorophyll fluorescence (77 K) and photon yield of O2-evolution in leaves of higher plants. Planta 171: 171–184
Dietz K-J, Schreiber U and Heber U (1985) The relationship between the redox state of QA and photosynthesis in leaves at various carbon-dioxide, oxygen and light regimes. Planta 166: 219–226
Gong H and Nilsen S (1989) Effect of temperature on photoinhibition of photosynthesis, recovery and turnover of the 32kD chloroplast protein in Lemna gibba. J Plant Physiol 135: 9–14
Greer DH, Ottander C and Öquist G (1991) Photoinhibition and recovery of photosynthesis in intact barley leaves at 5 and 20°C. Physiol Plant 81: 203–210
Havaux M, Strasser RJ, Greppin H (1991) A theoretical and experimental analysis of the qP and qN coefficients of chlorophyll fluorescence quenching and their relation to photochemical and non-photochemical events. Photosynth Res 27: 41–55
Joliot P, Bennoun P and Joliot A (1973) New evidence supporting energy transfer between photosynthetic units. Biochim Biophys Acta 305: 317–328
Krause GH (1988) Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol Plant 74: 566–574
Kyle DJ, Ohad I and Arntzen CJ (1984) Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci USA 81: 4070–4074
Kyle DJ (1987) The biochemical basis for photoinhibition of Photosystem II. In: Kyle DJ, Osmond CB and Arntzen CJ (eds) Topics in Photosynthesis, Vol 9, pp197–226. Elsevier, Amsterdam
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Markgraf T and Berry J (1990) Measurement of photochemical and non-photochemical quenching: Correction for turnover of PS II during steady-state photosynthesis. In: Baltscheffsky M (ed) Current Research in Photosynthesis, Vol 4, pp279–282. Kluwer Academic Publishers, Dordrecht
Melis A and Anderson JM (1983) Structural and functional organization of the photosystems in spinach chloroplasts. Antennae size, relative electron transport capacity and chlorophyll composition. Biochim Biophys Acta 724: 473–484
Nechushtai R and Nelson N (1985) Biogenesis of Photosystem I reaction centre during greening of oat, bean and spinach leaves. Plant Mol Biol 4: 377–384
Ögren E (1988) Photoinhibition of photosynthesis in willow leaves under field conditions. Planta 175: 229–236
Ögren E (1991) Prediction of photoinhibition of photosynthesis from measurements of fluorescence quenching components. Planta 184: 538–544
Öquist G and Huner NPA (1991) Effects of cold acclimation and leaf orientation on the susceptibility of photosynthesis to photoinhibition in Scots pine and in winter and spring cereals. Func Ecol 5: 91–100
Öquist G and Huner NPA (1992) Frost-hardening induced resistance to photoinhibition of photosynthesis in winter rye depends on an increased capacity of photosynthesis. Planta (in press)
Öquist G, Greer DH and Ögren E (1987) Light stress at low temperature. In: Kyle DJ, Osmond CB and Arntzen CJ (eds) Topics in Photosynthesis, Vol 9, pp67–87. Elsevier, Amsterdam
Öquist G, Chow WS and Anderson JM (1992) Photoinhibition of photosynthesis represents a mechanism for the long-term regulation of Photosystem II. Planta 186: 450–460
Osmond CB (1981) Photorespiration and photoinhibition. Some implications for the energetics of photosynthesis. Biochim Biophys Acta 639: 77–89
Papageorgiou G (1975) Chlorophyll fluorescence: An intrinsic probe of photosynthesis. In: Govindjee (ed) Bioenergetics of Photosynthesis (Cell biology) pp320–371. Academic Press Inc, New York
Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol 35: 15–44
Richter M, Rühle W and Wild A (1990) Studies on the mechanism of Photosystem II photoinhibition. II. The involvement of toxic oxygen species. Photosynth Res 24: 237–243
Schöner S, Foyer C, Lelandais M and Krause GH (1989) Increase in activities of scavengers for active oxygen in spinach related to cold acclimation in excess light. In: Baltscheffsky M (ed) Current Research in Photosynthesis, Vol II, pp483–486. Kluwer Academic Publishers, Dordrecht
Shipton CA and Barber J (1991) Photoinduced degradation of the D1 polypeptide in isolated reaction centers of Photosystem II: Evidence for an autoproteolytic process triggered by the oxidizing side of the photosystem. Proc Natl Acad Sci USA 88: 6691–6695
Tischer W and Strotmann H (1977) Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron transport. Biochim Biohys Acta 460: 113–125
Towbin H, Staehelin T and Cordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gel to nitrocellulose sheets. Procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4353
van Kooten O and Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25: 147–150
Virgin I, Styring S and Andersson B (1988) Photosystem II disorganization and manganese release after photoinhibition of isolated spinach thylakoid membranes. FEBS Lett 233: 408–412
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ottander, C., Hundal, T., Andersson, B. et al. Photosystem II reaction centres stay intact during low temperature photoinhibition. Photosynth Res 35, 191–200 (1993). https://doi.org/10.1007/BF00014750
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00014750