Abstract
Standard methods for quantifying GHG emissions from soils tend to use either micrometeorological or chamber-based measurement approaches. The latter is the most widely used technique, since it can be applied at low costs and without power supply at remote sites to allow measurement of GHG exchanges between soils and the atmosphere for field trials. Instrumentation for micrometeorological measurements meanwhile is costly, requires power supply and a minimum of 1 ha homogeneous, flat terrain. In this chapter therefore we mainly discuss the closed chamber methodology for quantifying soil GHG fluxes. We provide detailed guidance on existing measurement protocols and make recommendations for selecting field sites, performing the measurements and strategies to overcome spatial variability of fluxes, and provide knowledge on potential sources of errors that should be avoided. As a specific example for chamber-based GHG measurements we discuss sampling and measurement strategies for GHG emissions from rice paddies.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Paddy Soil
- Chamber Headspace
- Micrometeorological Measurement
- Soil Environmental Condition
- National Ecological Observatory Network
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Introduction
Microbial processes in soils, sediments, and organic wastes such as manure are a major source of atmospheric greenhouse gases (GHG). These processes create spatially as well as temporally heterogeneous sources or sinks. Consequently, a thorough understanding of the underlying processes and a quantification of spatiotemporal dynamics of sinks and sources are the bases for (a) developing GHG inventories at global, national, and regional scales, (b) identifying regional hotspots and (c) developing strategies for mitigating GHG emissions from terrestrial, specifically agricultural systems.
At the ecosystem scale, biosphere–atmosphere fluxes of CO2, CH4, and N2O are bi-directional, i.e., what is observed is a net flux of production and consumption processes (e.g., CO2: photosynthesis and autotrophic and heterotrophic respiration; CH4: methanogenesis and methane oxidation; N2O: nitrification and de-nitrification as source processes and de-nitrification as a sink process). The same is true for soil–atmosphere exchange processes , though, with regard to CO2, often only respiratory fluxes are measured.
Approximately 2/3 of all N2O emissions are linked to soil and manure management (Fowler et al. 2009; IPCC 2013). For CH4 as well, soils and organic wastes strongly influence atmospheric CH4 concentrations. It is estimated that wetland and paddy soils represent approximately 1/3 of all sources for atmospheric CH4 (Fowler et al. 2009). On the other hand, well-aerated soils of natural and semi-natural ecosystems—and to a lesser extent soils of agroecosystems—are sinks for atmospheric CH4 , removing approximately 20–45 Tg yr−1 of CH4 from the atmosphere (Dutaur and Verchot 2007), which corresponds to approximately 6–8 % of all sinks for atmospheric CH4 (Fowler et al. 2009). For CO2, soils are a major source due to autotrophic (plant root) and heterotrophic (microbial and soil fauna breakdown of organic matter) respiration. However, at the ecosystem scale, soils can act as net sinks as well as sources for CO2, since at this scale plant primary production (CO2 fixation from the atmosphere by photosynthesis), litter input to soils as well as respiratory fluxes are considered. It is well established that soils to a depth of 1 m globally store approximately three times the amount of carbon currently found in the atmosphere (Batjes 1996; IPCC 2013). Thus, land use and land management changes, as well as changes in climate affect plant primary production and fluxes of litter to the soil and soil organic matter mineralization dynamics. This can either result in a mobilization of soil C and N stocks, or, with adequate management, turn soils into C sinks. The latter is an essential process for removal of atmospheric CO2 and climate protection and has been called the “recarbonization ” of our terrestrial ecosystems (Lal 2009).
Due to the mostly microbiological origin of soil, sediment, and organic waste GHG emissions, changes in environmental conditions directly affect the exchange of GHG between terrestrial systems and the atmosphere (Butterbach-Bahl and Dannenmann 2011). Changes in temperature affect enzyme activities, while changes in redox conditions—as influenced by soil aeration fluctuations as a consequence of changes in soil moisture—can favor sequentially different microbial processes. For example, field irrigation and flooding as a standard management for rice paddies results in anaerobic soil conditions, thereby slowing down and stopping aerobic decomposition processes, while sequentially initializing a series of microbial processes that use elements and compounds other than oxygen as an electron acceptor: first NO3 − (denitrification), followed by SO4 − and Fe3+ and Mn3+/4+ reduction, before finally CH4 is produced as a product of organic matter degradation under strictly anaerobic conditions by methanogens (Conrad 1996).
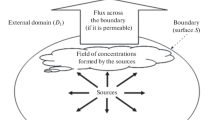
Environmental conditions not only change naturally across days, seasons, and years as a consequence of diurnal and seasonal temperature and rainfall regimes, but also due to management of agricultural (forest with regard to plantations) land, as was explained above with the example of flooding of paddy fields. Changes in environmental conditions affect the activity of the microbial community as well as that of plants, and consequently, the associated GHG production and consumption processes. Thus, GHG emissions from soils show a rather pronounced temporal variability on short (diurnal) and longer (days to weeks and years) timescales (e.g., Luo et al. 2012). Moreover, environmental conditions also vary spatially because soil conditions, plant cover, land management and thus, nutrient availability, soil aeration and microbial community composition, also change across micro- (e.g., soil matrix) to landscape and continental scales. As a result, GHG fluxes also vary considerably across spatial scales, making it necessary to develop a solid sampling strategy to target measurement sites, i.e., determine which sites are representative for the landscape one would like to work in, to estimate GHG fluxes and develop strategies to mitigate those emissions. Targeting (Chap. 2 of these guidelines) is a cornerstone to allow meaningful upscaling to landscape and higher spatial scales. But targeting already starts at the measurement site, since decisions have to be made about where (and when) to place chambers for flux measurements (Fig. 4.1a).
This chapter does not aim to provide a cookbook of how to measure soil and GHG fluxes. Plenty of work has been published on this topic, filling bookshelves and libraries (see e.g., Table 4.1). Here, we provide guidance to the relevant literature and highlight potential problems that might come up when designing a GHG measurement program (Fig. 4.1) rather than explain the sampling procedures in detail. We also provide examples of how to overcome problems in the context of GHG measurements for smallholder systems.
4.2 What Technique Is Most Suitable for Measuring Biosphere–Atmosphere Exchange Processes of GHGs?
The two most commonly used techniques for measuring fluxes between terrestrial ecosystems and the atmosphere are: (a) enclosure-based (chamber) measurements (manual or automated) and (b) micrometeorological measurements (e.g., eddy covariance or gradient methods), or a combination of both (Denmead 2008). The choice of the measurement technique itself is largely driven by resource investment, demand, and by the research question.
4.2.1 Micrometeorological Measurements
Use of micrometeorological techniques requires homogenous fields with a significant fetch (>1 ha) that should not be influenced by buildings, trees, slopes, etc. Land use, land management, vegetation, and soil properties should be homogeneous for the direct fetch area, but also for the wider area. Typically these techniques are applied in flat terrain with large, homogeneous land use, such as pasture, grassland, maize, or wheat monocrops, forests, or tree plantations. Capital costs of micrometeorological measurements of GHG fluxes are high, since the required sensors (3D wind field, fast-response gas analyzers) plus auxiliary instruments (meteorological station, mast, etc.) for flux measurements at one site, cost around 60,000–80,000 USD for CO2 and energy fluxes alone. Adding other components, such as CH4 (open path sensors are available) and N2O (requiring laser spectroscopy instruments), requires a significant additional investment in instruments, starting from 30,000 to 40,000 USD per gas. Energy supply for the instruments (if not only focused on open path CO2/H2O/CH4 technology) is another constraint that should be considered. The two most prominent global networks for multi-site and multi-species observations of biosphere–atmosphere-exchange of GHGs using micrometeorological methodologies are the National Ecological Observatory Network (NEON) in the USA (http://neoninc.org/) and the Integrated Carbon Observation Network (ICOS) in Europe (http://www.icos-infrastructure.eu/?q=node/17). Both networks offer information, processing tools for calculating fluxes and experts for providing support for designing, establishing, and running micrometeorological measurements.
Micrometeorological techniques for assessing GHG exchange are not recommended for smallholder systems due to the complexity of land uses and land management, small-scale gradients in soil fertility, and complex crop rotations with intercropping (Chikowo et al. 2014).
Some literature for a first reading on micrometeorological techniques is listed in Table 4.1.
4.2.2 Chamber Measurements
This technique allows measurements of GHG fluxes at fine scales, with chambers usually covering soil areas <1 m2, and are thus much better suited for smallholder farming systems. They can be operated manually or automatically (Breuer et al. 2000). Chamber measurements are rather simple and therefore the most common approach for GHG measurements since they allow gas samples to be stored for future analysis and, with the exception of automated systems, they do not require power supply at the site. In contrast with micrometeorological approaches, chambers are suitable for exploring treatment effects (e.g., fertilizer and crop trials) or effects of land use, land cover, or topography on GHG exchange. However, care must be used in order to obtain accurate data, since installation of the chamber disturbs environmental conditions and measured fluxes might not necessarily reflect fluxes at adjacent sites if some precautions are not considered (see Sect. 5.2.1 below).
There are two types of chambers: dynamic and static chambers. For dynamic chambers the headspace air is exchanged at a high rate (>1–2 times the chamber’s volume per minute) and fluxes are calculated from the difference in gas concentrations at the inlet and outlet of the chambers multiplied by the gas volume flux, thereby considering the area which is covered by the chamber (Butterbach-Bahl et al. 1997a, b). Static chambers are gas-tight, without forced exchange of the headspace gas volume, and are usually vented to allow pressure equalization between the chamber’s headspace and the ambient air pressure (e.g., Xu et al. 2006). The volume of the “vent tube” should be greater than the gas volume taken at each sampling time.
Two situations call for using dynamic chambers: first, when measuring reactive gas fluxes such as soil NO emissions, and when there is a need to minimize the bias of changes in headspace air concentrations on the flux (Butterbach-Bahl et al. 1997a, b). The second point is important, as significant deviations of chamber headspace gas concentrations from ambient air concentrations affect the exchange process between soils and the atmosphere itself, since the flux at the soil–atmosphere interface is the result of simultaneous production and consumption processes. For example, if N2O concentration in the chamber headspace is much higher than atmospheric concentrations, microbial consumption processes are stimulated. Moreover, since emissions are mainly driven by diffusion and gas concentration gradients, significant increases/decreases in headspace concentrations of the gas of interest will slow down/accelerate the diffusive flux. Both mechanisms finally result in a deviation of the flux magnitude from undisturbed conditions (Hutchinson and Mosier 1981). It is important to be aware of this, though for practical reasons it is partly unavoidable because the precision of the analytical instruments used for gas flux measurements, such as electron capture detectors (ECDs) and gas chromatography, is insufficient to allow for dynamic chamber measurements. However, there are methods to cope with this problem, such as using non-linear instead of linear models to calculate fluxes as measured with static chamber technique (e.g., Kroon et al. 2008; Table 4.1), using quantum cascade lasers (QCLs) in the field (fast box; Hensen et al. 2006) and in general by minimizing chamber closure time as much as possible. Chamber closure time is dictated not only by the magnitude of the gas flux but also by the chamber height. Therefore, in agricultural systems where plants need to be included for representative measurements, it is suggested to use chambers which can be extended by sections according to plant growth (Barton et al. 2008).
Static chambers are usually mounted on a frame which should be inserted (approximately 0.02–0.15 m) at least a week before first flux measurements to overcome initial disturbances of soil environmental conditions due to the insertion of the frame. Once the chamber is closed gas-tight on the frame, headspace concentrations start to change, either increasing if the soil is a net source (e.g., for CO2—Fig. 4.2), or decreasing if the soil is functioning as a net sink (e.g., CH4 uptake by upland soils). For accurate calculation of gas flux, a minimum of four gas samples from the chamber headspace across the sampling interval (e.g., 0, 10, 20, 30 min following closure) is recommended (Rochette 2011).
Theoretical evolution of the concentration of a gas being emitted from the soil upon use of a static chamber. Concentration of the gas above the soil surface (black line) remains at a relatively constant level; at the moment when the chamber is closed (left arrow), the concentration in its headspace begins to rise. Along the closing period of the chamber, several gas samples are taken (black squares) and subsequently the concentration is determined, e.g., by use of gas chromatography. Right after opening the chamber (right arrow) concentration above soil surface returns to atmospheric background levels. Soil GHG emissions are most commonly calculated from the linear increase of the headspace gas concentration during the chamber closing period (red line), the volume of the chamber, the area of the soil covered by the chamber, as well as air temperature, air pressure, and molecular weight of the molecule under investigation (see e.g., Butterbach-Bahl et al. 2011). It should be noted that changes in gas concentration upon chamber closure can significantly deviate from linearity, showing, e.g., saturation effects. In all cases it should be tested if non-linear flux calculation methods do not fit the better observed changes in chamber headspace concentrations with time (see e.g., Pedersen et al. 2010)
Gas flux measurements with static and dynamic chambers have been described extensively and Table 4.1 provides an overview of recommended literature, while Fig. 4.1 indicates important considerations when using chamber methodology. Static chambers can not only be used for measurement of soil N2O and CH4 and CO2 respiratory fluxes, but also for measuring net ecosystem exchange of carbon dioxide. The latter requires the use of transparent chambers and consideration of corrections for photosynthetically active radiation and temperature inside and outside the chamber (Wang et al. 2013).
4.2.2.1 Chambers and Changes in Environmental Conditions
Closing a chamber gas-tight from the surrounding environment immediately affects a number of boundary conditions. The pressure inside the chamber might differ from outside, because when chambers are gas-tight and exposed to sunlight, the temperature of the headspace air increases so that air pressure inside in the chamber increases too. Both factors affect the gas exchange between the soil and the air. Thus, chambers should be heat insulated and opaque (except for the determination of net ecosystem respiration; see Zheng et al. 2008a, b) and a vent should be used (see Hutchinson and Livingston 2001) to equilibrate pressure differences between ambient and headspace air. Upon chamber closure of transparent non-insulated chambers exposed to direct sunlight, headspace temperature might increase by 10–20 °C within 20 min. Insulated chambers will also show a slight increase in soil headspace temperature. This affects microbial as well as plant respiratory activity. Therefore, minimizing closure times is necessary not only to minimize the effects of changing headspace gas concentrations on diffusive fluxes as described above, but to minimize temperature changes as well as (Table 4.1). One should therefore calculate the minimum flux that can be detected with the analytical instrument to be used and adjust the closure time accordingly. If possible, limit closure time to a maximum of 30–45 min. If automated chamber systems are used, change positions weekly or at 2-week intervals to minimize effects on soil environmental conditions, in particular soil moisture. Chambers have been shown to reduce soil moisture even if they open automatically during rainfall (Yao et al. 2009).
4.2.2.2 Chambers and Spatial Variability of GHG Fluxes
Soil environmental conditions change on a small scale due to differences in (a) bulk density resulting from machine use or livestock grazing, (b) texture as a consequence of soil genesis, (c) management (rows, inter-rows, cropping), (d) temperature (plant shading), (e) soil moisture (e.g., groundwater distances or as an effect of texture differences), (f) soil organic carbon (heterogeneous distribution of harvest residues) or (g) rooting depth and distribution (with effects on soil microbial diversity, activity, and distribution) (see Fig. 4.1a). For example, urine or feces dropping by livestock on rangeland or manure application to cropland has been shown to increase spatial and temporal variability of fluxes, since at plot scale not every patch responds equally to increased availability of substrate for microbial N and C turnover processes due to small-scale differences in soil properties, soil environmental conditions, and microbial activity and diversity. Overcoming spatial variability effects on GHG fluxes is a major challenge, specifically for highly diverse smallholder systems. The problem can be addressed by proper sampling design (Fig. 4.1) (see e.g., Davidson et al. 2002) or by using the gas pooling technique (Arias-Navarro et al. 2013) (Fig. 4.3).
The concept of gas pooling. (a) Gas pooling across chambers for a given sampling time, (b) gas sample mixing within the syringe, (c) transfer of the gas sample to a vial, (d) four vials for four sampling times and five chambers, (e) air sample analysis via gas chromatography (for further details see Arias-Navarro et al. 2013)
Proper sampling design in this context requires firstly that the landscape should be stratified into a number of separate categories. This stratification needs to include geophysical information as well as management activities. Also, in order to understand the drivers of the management decisions, it is critical to collect the political and socioeconomic climate of the various farms. The sampling approach can then concentrate measurement activities on emission hotspot and leverage points to capture heterogeneity and account for the diversity and complexity of farming activities (Rosenstock et al. 2013).
The gas pooling technique is similar to what is usually done for soil or water analyses. The principal idea of gas pooling is to generate a composite air sample out of the headspace of several chambers (Fig. 4.3). The chamber headspace is sampled at least four times across the closure period as is usually done, but gas samples at time 0, 10, 20, or 30 min are combined for several chambers of each individual sampling time (Arias-Navarro et al. 2013). As a consequence, information on the spatial variability is lost, but can be regained if on some sampling days, fluxes of the chambers are measured individually. This technique allows installation of a significantly higher number of chambers without increasing the amount of gas samples to be analyzed.
4.3 Measurement of GHG Fluxes in Rice Paddies
Due to its importance as a source for atmospheric CH4 we specifically discuss measurement of GHG fluxes in rice paddies in more detail. Unlike other field crops, rice is usually grown in flooded fields. The standing water creates anaerobic conditions in the soil that allows growth of a certain class of microorganisms (methanogenic archaea) that use simple carbon compounds (e.g., CO2 or acetate) as electron donors and produce methane in anaerobic respiration. Methane oxidation , on the other hand, does occur but only in the uppermost mm of flooded paddy soil or in the rhizosphere—due to radial O2 losses of rice roots (Butterbach-Bahl et al. 1997a, b)—and during unflooded periods. Since methanogenic archaea are extremely sensitive to oxygen and immediately stop CH4 production while stimulating CH4 oxidation, drainage of rice fields is an attractive mitigation option.
Methane is the most important GHG in rice production systems and has some implications on the chamber design and sampling time. Nitrous oxide emissions are generally low in flooded fields but increase with drainage. However, this increase in N2O emissions does not offset the mitigation effect that dry field conditions have on CH4 emissions (Sander et al. 2014).
Overall, requirements for GHG measurements in flooded rice production systems (dominated by CH4 emissions) are partly different from measurement in upland systems , which has some important implications on the chamber design and general sampling procedure (Table 4.2).
4.3.1 Rice Chamber Design and General Procedure (See Also Table 4.2)
Methane that is produced in the soil has three different emission pathways to the atmosphere: (1) diffusion through the water layer, (2) ebullition (bubbling), and (3) transport through the aerenchyma of the rice plants. The largest share of emitted methane (up to 90 %) is in fact transported through the rice plant itself (Wassmann et al. 1996; Butterbach-Bahl et al. 1997a, b), which makes it indispensable to include rice plants into the closed chamber (→ chamber height >1 m). This also applies to any measurements of wetland GHG fluxes, since plant-mediated transport is of critical importance here as well. The chamber base (the part of the chamber that remains in the soil during the whole growing season) should be installed at least 1 day (better a week or more) before the start of the sampling campaign and should not be higher than ~20 cm (with 10 cm below and 10 cm above soil surface) in order to minimize an effect on plant growth. To account for variability within the field, each chamber should include at least 4 rice plants or 4 “hills” in a transplanted system and an area of average plant density in a seeded system, resulting in a chamber area of ≥0.16 m2. Note that due to the flooded field conditions, the chamber base in rice systems should have holes (~2 cm above soil surface) to allow water exchange between the chamber inside and the field. This hole or holes must be closed before sampling in case irrigation water level falls and the hole(s) is above the water layer.
Movement in the wet paddy soil can potentially cause gas bubbles to evolve and impede undisturbed gas sampling. Therefore, installation of boardwalks in the field is highly recommended. Exposure to high air temperatures and high solar radiation often characterize rice paddies and so it is in especially crucial to ensure that the plants inside the chambers are not damaged by heat stress during sampling. Therefore, the chamber material should be reflective or white or the chamber should be equipped with proper insulation. Since the gas volume in the closed chamber changes due to temperature increase and samples being taken, chambers should have a vent to allow equilibration with outside air pressure.
4.3.2 Time of Day of Sampling
Methane emissions typically follow a distinct diurnal variation following changes in soil temperature (Neue et al. 1997), i.e., low emissions during night time that increase after sunrise, peak around noon to early afternoon and decrease again thereafter. Therefore the timing of gas sampling is of great importance in order to measure as close as possible to a time representing a daily average flux rather than at times leading to over or underestimation of fluxes. Minamikawa et al. (2012) found that methane fluxes around 10 a.m. were closest to the daily mean CH4 flux in temperate regions. Similar assumptions are likely valid for tropical and subtropical regions. However, we recommend measuring region-specific diurnal emission patterns at least three times during the growing season of rice and based on the observed diurnal pattern to decide on the best sampling time. Alternatively, measuring diurnal soil temperature profiles at 5-cm depth can provide reasonable estimations of the time of day with mean methane emission because soil temperature and CH4 flux are closely related.
4.3.3 Sampling Frequency
The precision of cumulative seasonal GHG emissions largely depends on the sampling frequency. Minamikawa et al. (2012) found that sampling once a week for flooded rice in temperate regions resulted in an accurate estimation of total emissions. Buendia et al. (1998) proposed a more flexible sampling schedule of 10-day intervals in the beginning of the growing season, 20-day intervals in the middle and 7-day intervals at the end of the season in tropical environments and came up with similarly accurate seasonal emission estimates.
It is important to note that more frequent sampling is necessary during dry periods of rice cultivation as methane emissions from paddy soils with a high clay content show a sharp peak when drainage is applied (Lu et al. 2000) and nitrous oxide emissions increase during dry periods (Jiao et al. 2006). In order to have complete flux information of an area, some gas samples should also be taken between two cropping seasons.
4.4 Analytical Instruments Used for Chamber Measurements
When using the static chamber approach, several analytical instruments can be used for determining GHG concentrations in the sample air, either directly in the field or, following storage of headspace gas samples in vials or gas-tight syringes, at a later time in the laboratory. The latter always requires that the gas-tightness of the vials/ syringes is tested regularly.
4.4.1 Gas Chromatography
Instruments used for gas sample analysis rely on different operational principles. Gas chromatography (GC ) is the most commonly used analytical technique when determining GHG concentrations in gas samples from chambers (e.g., Keller et al. 1986; Kiese and Butterbach-Bahl 2002; Kelliher et al. 2012). Usually, 1–3 mL of air sample is injected into the gas chromatograph and the different compounds are separated on an analytical column (e.g., Hayesep N for N2O, 3 m, 1/8″) for detection with various detectors. For N2O a 63Ni Electron Capture Detector (ECD) is commonly used. The ECD should be operated at between 330 and 350 °C, since the N2O sensitivity is highest and the cross-sensitivity to CO2 is lowest in this range. However, there is still a cross-sensitivity to CO2 if N2 is used as sole carrier and purge gas (Zheng et al. 2008a, b; Wang et al. 2010). No cross-sensitivity exits if Argon/CH4 is used as carrier gas or if the ECD cell is purged with a gas mixture of 5 % CO2 in N2 (Wang et al. 2010). Another possibility to eliminate the cross-sensitivity of N2O and CO2 is to use a pre-column filled with Ascarite (coated NaOH), which scrapes the CO2 from the gas-stream. However, pre-columns need to be changed frequently (approximately 2-week intervals) due to saturation and capturing of air sample moisture.
Another critical point is that if gas chromatographs with ECD are used for concentration measurements, the signal to concentration ratio might deviate from a linear response if—in the case of N2O—sample air concentrations are significantly >700 ppbv. Therefore, a check of the linearity of the signal to concentration ratio should be done for each instrument and gas under consideration.
For CH4 a flame ionization detector (FID) is normally used and, if a methanizer is introduced before the detector, CO2 can also be measured with a FID (or more standard: use of a thermal conductivity detector for CO2).
4.4.2 Spectroscopic Methods
Spectroscopic methods are becoming more and more prominent for measuring GHG fluxes between soils and the atmosphere by static chamber technique. A specific example is photoacoustic spectroscopy (PAS), with instruments being miniaturized to make them suitable for direct field use, e.g., allowing direct measurements of changes in chamber headspace N2O, CH4, or CO2 concentration with time following chamber closure (e.g., Leytem et al. 2011). PAS technique, as every spectroscopic method, is based on the principle that GHGs absorb light at a specific wavelength, here in the infrared spectra. The absorption is thereby directly linked to the concentration (Beer-Lambert law) and in the case of PAS, the absorption of the light or energy is converted into an acoustic signal, which is measured by a microphone. For chamber measurements in the field, the PAS instrument is usually connected to the chamber in a closed loop so that the air from the apparatus exhaust is returned to the chamber avoiding underpressure or dilution.
PAS instruments are becoming popular as an alternative to GC-technique due to portability, low maintenance, and ease-of-operation (Iqbal et al. 2012). In principle, commercially available PAS instruments, such as INNOVA (Lumasense Technologies) require a yearly calibration only and are “plug-and-play” instruments ready to be used in the field. However, because GHGs and water vapor have multiple absorption bands across the measuring spectra, such instruments are prone to interferences. Recently, Rosenstock et al. (2013) showed that for INNOVA instruments N2O concentration measurements were non-linearly affected by water content and CO2. Comparable results were already reported by Flechard et al. (2005), though only a few researchers have noted the problems that might be associated with the use of PAS. The manufacturers claim that the INNOVA software accounts for cross interferences, but corrections do not seem to work sufficiently while testing several instruments (Rosenstock et al. 2013). Furthermore, there is also evidence that ambient air temperature affects the electronics and thus, the reliability of measured GHG concentrations (Rosenstock et al. 2013), when using PAS under field conditions. Specifically for N2O, measured concentrations varied up to 100 % depending on environmental conditions (Rosenstock et al. 2013). Also the precision and accuracy of CH4 measurements seems to be rather low, with deviations in concentration of nearly 400 % for calibration gases (Rosenstock et al. 2013). As it stands now, it is advisable to question the use of INNOVA instruments for CH4 as well as for N2O measurements in particular by using the instrument for simultaneous measurements of multiple gas species.
Other techniques may include tunable diode lasers (TDL), quantum cascade lasers (QCL), Fourier transform infrared spectroscopy (FTIR) or cavity ring-down spectroscopy (CRDS). Instruments using these spectroscopic techniques usually operate under high vacuum and, thus, a continuous air flow through the instrument is required. Therefore, instruments need to be at the study site and physically connected to chambers. Though these instruments are still quite expensive (e.g., compared to GC) they are becoming more and more robust and suitable for field applications. However, a constant (use of UPS is suggested) main power supply is still needed and checks for cross-sensitivity should be a standard procedure in the laboratory.
4.4.3 Auxiliary Measurements
As described earlier in this chapter, spatiotemporal patterns of GHG fluxes are closely linked to changes in environmental conditions (see also Fig. 4.1). Therefore, GHG flux measurements are rather useless if environmental parameters such as soil and vegetation properties and management are not monitored at the same time, since these factors significantly affect fluxes. This necessarily also includes the quantification of soil C and N stocks, as for example application of animal manure to arable fields and rangeland has been shown to significantly increase soil carbon stocks (Maillard and Angers 2014), which need to be considered when calculating the GHG balance of a given system. Moreover, since GHG flux measurements are expensive and can’t be repeated everywhere, models need to be developed, tested, and finally used for estimating fluxes at landscape, regional, and global scale as well as for exploring mitigation options at multi-year scales or for predicting climate change feedbacks on biosphere–atmosphere exchange processes. Comprehensive datasets, including both flux measurements and detailed information on soil and vegetation properties and management are prerequisites for model development and testing. Surprisingly such datasets are still scarce, because either flux measurements do not meet the required measuring standards or the needed auxiliary measurements and site information are not monitored or reported.
Since responsibilities for GHG flux and auxiliary measurements are often split between collaborators, there is a need to clarify personal responsibility of data provision prior to the start of measurements. Rochette and Eriksen-Hamel (2007) reviewed published N2O flux data and developed a minimum set of criteria for chamber design and methodology. According to their evaluation of 365 studies, there was low to very low confidence in reported flux values in about 60 % of the studies due to poor methodologies or incomplete reporting. Thus, it is necessary to improve not only the quality of flux measurements, but also the reporting of soil and vegetation properties and management. See Fig. 4.1 for suggested variables for measurement.
4.5 Conclusions
Micrometeorological or chamber-based techniques can be used for the quantification of biosphere–atmosphere exchange processes of GHGs. In view of the diversity and patchiness of land uses and land management associated with smallholder agriculture, chamber-based methods, specifically the closed (static) chamber approach, is recommended. Overcoming spatial and temporal variability of fluxes remain an issue, and should be addressed by a well-designed sampling scheme including landscape targeting of measuring sites (see Rufino et al. this book), targeting of chamber placement at field and plot scale (Fig. 4.1), running of at least 3–5 replicates per plot to address small-scale variability (and possibly use of the gas pooling technique, Fig. 4.3), flux measurements in weekly intervals over a period of at least 1 year and detailed documentation of environmental conditions and field activities (Fig. 4.1). This will ensure that all data can finally be used for modeling and upscaling. Quality control and quality assurance remains an issue at all steps, also with regard to gas analytics. Probably the most efficient way for a researcher to familiarize him- or herself with gas flux measurement techniques is a longer stay with a recognized research group.
References
Arias-Navarro C, Díaz-Pinés E, Kiese R, Rosenstock RS, Rufino MC, Stern D, Neufeldt H, Verchot LS, Butterbach-Bahl K (2013) Gas pooling: a sampling technique to overcome spatial heterogeneity of soil carbon dioxide and nitrous oxide fluxes. Soil Biol Biochem 67:20–23
Bain WG, Hutyra L, Patterson DC, Bright AV, Daube BC, Munger JW, Wofsy SC (2005) Wind-induced error in the measurement of soil respiration using closed dynamic chambers. Agric For Meteorol 131:225–232
Barton L, Kiese R, Gatter D, Butterbach-Bahl K, Buck R, Hinz C, Murphy DV (2008) Nitrous oxide emissions from a cropped soil in a semi-arid climate. Glob Chang Biol 14:177–192
Batjes NH (1996) Total carbon and nitrogen in the soils of the world. Eur J Soil Sci 47:151–163
Breuer L, Papen H, Butterbach-Bahl K (2000) N2O-emission from tropical forest soils of Australia. J Geophys Res 105:26353–26378
Buendia LV, Neue HU, Wassmann R, Lantin RS, Javellana AM, Arah J, Wang Z, Wanfang L, Makarim AK, Corton TM, Charoensilp N (1998) An efficient sampling strategy for estimating methane emission from rice field. Chemosphere 36:395–407
Butterbach-Bahl K, Dannenmann M (2011) Denitrification and associated soil N2O emissions due to agricultural activities in a changing climate. Curr Opin Environ Sustain 3:389–395
Butterbach-Bahl K, Papen H, Rennenberg H (1997a) Impact of gas transport through rice cultivars on methane emission from rice paddy fields. Plant Cell Environ 20(9):1175–1183
Butterbach-Bahl K, Gasche R, Breuer L, Papen H (1997b) Fluxes of NO and N2O from temperate forest soils: impact of forest type, N deposition and of liming on the NO and N2O emissions. Nutr Cycl Agroecosyst 48:79–90
Butterbach-Bahl K, Kiese R, Liu C (2011) Biosphere-atmosphere exchange of CH4 in terrestrial systems. In: Rosenzweig AC, Ragsdale SW (eds) Methods in enzymology, vol 49. Academic, New York, pp 271–287
Chikowo R, Zingore S, Snapp S, Johnston A (2014) Farm typologies, soil fertility variability and nutrient management in smallholder farming in Sub-Saharan Africa. Nutr Cycl Agroecosyst 100:1–18
Conen F, Smith KA (1998) A re-examination of closed chamber methods for the measurement of trace gas emissions from soils to the atmosphere. Eur J Soil Sci 49:701–707
Conen F, Smith KA (2000) An explanation of linear increases in gas concentration under closed chambers used to measure gas exchange between soil and the atmosphere. Eur J Soil Sci 51:111–117
Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60:609–640
Davidson EA, Savage K, Verchot LV, Navarro R (2002) Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agric For Meteorol 113:21–37
De Klein CAM, Harvey MJ (2012) Nitrous oxide chamber methodology guidelines. Global Research Alliance, Ministry of Primary Industries, New Zealand, 146p. Available via DIALOG: www.globalresearchalliance.org/research/livestock/activities/nitrous-oxide-chamber-methodology-guidelines/
Denmead OT (2008) Approaches to measuring fluxes of methane and nitrous oxide between landscapes and the atmosphere. Plant Soil 309:5–24
Dutaur L, Verchot LV (2007) A global inventory of the soil CH4 sink. Glob Biogeochem Cycles 21(4), GB4013
Flechard C, Neftel A, Jocher M, Ammann C, Fuhrer J (2005) Bi-directional soil/atmosphere N2O exchange over two mown grassland systems with contrasting management practices. Glob Chang Biol 11:2114–2127
Fowler D, Pilegaard K, Sutton MA, Ambus P, Raivonen M, Duyzer J, Simpson D, Fagerli H, Fuzzi S, Schjoerring JK, Granier C, Neftel A, Isaksen ISA, Laj P, Maione M, Monks PS, Burkhardt J, Daemmgen U, Neirynck J, Personne E, Wichink-Kruit R, Butterbach-Bahl K, Flechard C, Tuovinen JP, Coyle M, Gerosa G, Loubet B, Altimir N, Gruenhage L, Ammann C, Cieslik S, Paoletti E, Mikkelsen TN, Ro-Poulsen H, Cellier P, Cape JN, Horvath L, Loreto F, Niinemets U, Palmer PI, Rinne J, Misztal P, Nemitz E, Nilsson D, Pryor S, Gallagher MW, Vesala T, Skiba U, Brüggemann N, Zechmeister-Boltenstern S, Williams J, O’Dowd C, Facchini MC, de Leeuw G, Flossman A, Chaumerliac N, Erisman JW (2009) Atmospheric composition change: ecosystems—atmosphere interactions. Atmos Environ 43:5193–5267
Giltrap DL, Berben P, Palmada T, Saggar S (2014) Understanding and analyzing spatial variability of nitrous oxide emissions from a grazed pasture. Agric Ecosyst Environ 186:1–10
Groffman PM, Butterbach-Bahl K, Fulweiler RW, Gold AJ, Morse JL, Stander EK, Tague C, Tonitto C, Vidon P (2009) Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 93:49–77
Hensen A, Groot TT, Van den Bulk WCM, Vermeulen AT, Olesen JE, Schelde K (2006) Dairy farm CH4 and N2O emissions, from one square meter to the full farm. Agric Ecosyst Environ 112:146–152
Holst J, Liu C, Yao Z, Brüggemann N, Zheng X, Han X, Butterbach-Bahl K (2007) Importance of point sources on regional nitrous oxide fluxes in semi-arid steppe of Inner Mongolia, China. Plant and Soil 296:209–226
Hutchinson GL, Livingston GP (2001) Vents and seals in non-steady state chambers used for measuring gas exchange between soil and the atmosphere. Eur J Soil Sci 52:675–682
Hutchinson GL, Mosier AR (1981) Improved soil cover method for field measurement of nitrous oxide fluxes. Soil Sci Soc Am J 45:311–316
Hutchinson GL, Rochette P (2003) Non-flow through steady state chambers for measuring soil respiration: numerical evaluation of their performance. Soil Sci Soc Am J 67:166–180
International Atomic Energy Agency (IAEA) (1992) Manual on measurement of methane and nitrous oxide emissions from agriculture. In: Training Course Series No. 29, Vienna, pp 1–237
Iqbal J, Castellano MJ, Parkin TB (2012) Evaluation of photoacoustic infrared spectroscopy for simultaneous measurement of N2O and CO2 gas concentrations and fluxes at the soil surface. Glob Change Biol 19:327–336
Jiao Z, Hou A, Shi Y, Huang G, Wang Y, Chen X (2006) Water management influencing methane and nitrous oxide emissions from rice field in relation to soil redox and microbial community. Commun Soil Sci Plant Anal 37:1889–1903
Kebreab E, Clark K, Wagner-Riddle C, France J (2006) Methane and nitrous oxide emissions from Canadian animal agriculture: a review. Can J Anim Sci 86:135–158
Kroon PS, Hensen A, Van den Bulk WCM, Jongejan PAC, Vermeulen AT (2008) The importance of reducing the systematic error due to non-linearity in N2O flux measurements by static chambers. Nutr Cycl Agroecosyst 82:175–186
Kutzbach L, Schneider J, Sachs T, Giebels M, Nykänen H, Shurpali NJ, Martikainen PJ, Alm J, Wilmking M (2007) CO2 flux determination by closed chamber methods can be seriously biased by inappropriate application of linear regression. Biogeosciences 4:1005–1025
Lal R (2009) Sequestering atmospheric carbon dioxide. Crit Rev Plant Sci 28:90–96
Leytem AB, Dungan RS, Bjorneberg DL, Koehn AC (2011) Emissions of ammonia, methane, carbon dioxide, and nitrous oxide from dairy cattle housing and manure management systems. J Environ Qual 40:1383–1394
Liu C, Zheng X, Zhou Z, Han S, Wang Y, Wang K, Liang W, Li M, Chen D, Yang Z (2010) Nitrous oxide and nitric oxide emissions from irrigated cotton field in Northern China. Plant Soil 332:123–134
Livingston GP, Hutchinson GL, Spartalian K (2005) Diffusion theory improves chamber-based measurements of trace gas emissions. Geophys Res Lett 32, L24817
Lu WF, Chen W, Duan BW, Guo WM, Lu Y, Lantin RS, Wassmann R, Neue HU (2000) Methane emissions and mitigation options in irrigated rice fields in southeast China. Nutr Cycl Agroecosyst 58:65–73
Luo GJ, Brüggemann N, Wolf B, Gasche R, Grote R, Butterbach-Bahl K (2012) Decadal variability of soil CO2, NO, N2O, and CH4 fluxes at the Höglwald Forest, Germany. Biogeosciences 9:1741–1763
Luo J, Hoogendorn C, Van der Weerden T, Saggar S, De Klein C, Giltrap D, Rollo M, Rys G (2013) Nitrous oxide emissions from grazed hill in New Zealand. Agric Ecosyst Environ 181:58–68
Maillard É, Angers DA (2014) Animal manure application and soil organic carbon stocks: a meta-analysis. Glob Chang Biol 20:666–679
Minamikawa K, Yagi K, Tokida T, Sander BO, Wassmann R (2012) Appropriate frequency and time of day to measure methane emissions from an irrigated rice paddy in Japan using the manual closed chamber method. Greenhouse Gas Meas Manag 2(2–3):118–128
Neue HU, Wassmann R, Kludze HK, Wang B, Lantin RS (1997) Factors and processes controlling methane emissions from rice fields. Nutr Cycl Agroecosyst 49:111–117
Parkin TB (2008) Effect of sampling frequency on estimates of cumulative nitrous oxide emissions. J Environ Qual 37:1390–1395
Parkin TB, Venterea RT (2010) Sampling protocols. Chap 3 Chamber based trace gas flux measurements. In: Folett RF (ed) GRACEnet Sampling protocols, pp 3-1–3-39. www.ars.usda.gov/research/GRACEnet
Pattey E, Edwards G, Strachan IB, Desjardins RL, Kaharabata S, Wagner-Riddle C (2006) Towards standards for measuring greenhouse gas fluxes from agricultural fields using instrumented towers. Can J Soil Sci 86:373–400
Pedersen AR, Petersen SO, Schelde K (2010) A comprehensive approach to soil-atmosphere trace-gas flux estimation with static chambers. Eur J Soil Sci 61:889–902
Pihlatie M et al (2012) Comparison of static chambers to measure CH4 emissions from soils. Agric For Meteorol 171–172:124–136
Rochette P (2011) Towards a standard non-steady-state chamber methodology for measuring soil N2O emissions. Anim Feed Sci Technol 166(167):141–146
Rochette P, Eriksen-Hamel NS (2007) Chamber measurements of soil nitrous oxide flux: are absolute values reliable? Soil Sci Soc Am J 72:331–342
Rosenstock TS, Diaz-Pines E, Zuazo P, Jordan G, Predotova M, Mutuo P, Abwanda S, Thiong’o M, Buerkert A, Rufino MC, Kiese R, Neufeldt H, Butterbach-Bahl K (2013) Accuracy and precision of photoacoustic spectroscopy not guaranteed. Glob Chang Biol 19:3565–3567
Sander BO, Wassmann R (2014) Common practices for manual greenhouse gas sampling in rice production: a literature study on sampling modalities of the closed chamber method. Greenhouse Gas Meas Manag 4(1):1–13
Sander B, Wassmann R, Siopongco JDLC (2014) Water-saving techniques: potential, adoption and empirical evidence for mitigating greenhouse gas emissions from rice production. In: Hoanh CT, Smakhtin V, Johnston T (eds) Climate change and agricultural water management in developing countries. CABI Climate Change Series. CABI, Wallingford
Schütz H, Seiler W (1992) Methane flux measurements: methods and results. In: Andreae MO, Schimel DS (eds) Exchange of trace gases between terrestrial ecosystems and the atmosphere. Wiley, Chichester, pp 209–228
Smith KA, Dobbie KE (2001) The impact of sampling frequency and sampling times on chamber based measurements of N2O emissions from fertilized soils. Glob Chang Biol 7:933–945
Smith KA, Clayton H, McTaggart IP, Thomson PE, Arah JRM, Scott A, Goulding KWT, Monteith JL, Phillips VR (1996) The measurement of nitrous oxide emissions from soil by using chambers (and discussion). Philos Trans Phys Sci Eng 351:327–338
Venterea RT (2010) Simplified method for quantifying theoretical underestimation of chamber-based trace gas fluxes. J Environ Qual 39:126–135
Venterea RT, Baker JM (2008) Effects of soil physical non-uniformity on chamber-based gas flux estimates. Soil Sci Soc Am J 72:1410–1417
Venterea RT, Spokas KA, Baker JM (2009) Accuracy and precision analysis of chamber-based nitrous oxide gas flux estimates. Soil Sci Soc Am J 73:1087–1093
Wang Y, Wang Y, Ling H (2010) A new carrier gas type for accurate measurement of N2O by GC-ECD. Adv Atmos Sci 27:1322–1330
Wang K, Liu C, Zheng X, Pihlatie M, Li B, Haapanala S, Vesala T, Liu H, Wang Y, Liu G, Hu F (2013) Comparison between eddy covariance and automatic chamber techniques for measuring net ecosystem exchange of carbon dioxide in cotton and wheat fields. Biogeosciences 10:6865–6877
Wassmann R, Neue HU, Alberto MCR, Lantin RS, Bueno C, Llenaresas D, Arah JRM, Papen H, Seiler W, Rennenberg H (1996) Fluxes and pools of methane in wetland rice soils with varying organic inputs. Environ Monit Assess 42:163–173
Wood JD, Gordon RJ, Wagner-Riddle C (2013) Biases in discrete CH4 and N2O sampling protocols associated with temporal variation of gas fluxes from manure storage systems. Agric For Meteorol 171–172:295–305
Xu L, Furtaw MD, Madsen RA, Garcia RL, Anderson DJ, McDermitt DK (2006) On maintaining pressure equilibrium between a soil CO2 flux chamber and the ambient air. J Geophys Res 111:D08S10
Yao Z, Zheng X, Xie B, Liu C, Mei B, Dong H, Butterbach-Bahl K, Zhu J (2009) Comparison of manual and automated chambers for field measurements of N2O, CH4, CO2 fluxes from cultivated land. Atmos Environ 43:1888–1896
Zheng X, Xie B, Liu C, Zhou Z, Yao Z, Wang Y, Wang Y, Yang L, Zhu J, Huang Y, Butterbach-Bahl K (2008a) Quantifying Net Ecosystem Carbon Dioxide Exchange (NEE) of a short-plant cropland with intermittent chamber measurements. Glob Biogeochem Cycles 22, GB3031
Zheng X, Mei B, Wang Y, Xie B, Wang Y, Dong H, Xu H, Chen G, Cai Z, Yue J, Gu J, Su F, Zou J, Zhu J (2008b) Quantification of N2O fluxes from soil-plant systems may be biased by the applied gas-chromatography methodology. Plant Soil 311:211–234
Acknowledgments
This work was financed by the SAMPLES project of the Climate Change, Agriculture and Food Security (CCAFS) research program of CGIAR Centre and Research Programs. Eugenio Diaz-Pínés received additional funding from the EU-InGOS project.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, a link is provided to the Creative Commons license and any changes made are indicated.
The images or other third party material in this chapter are included in the work?s Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work?s Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2016 The Author(s)
About this chapter
Cite this chapter
Butterbach-Bahl, K., Sander, B.O., Pelster, D., Díaz-Pinés, E. (2016). Quantifying Greenhouse Gas Emissions from Managed and Natural Soils. In: Rosenstock, T., Rufino, M., Butterbach-Bahl, K., Wollenberg, L., Richards, M. (eds) Methods for Measuring Greenhouse Gas Balances and Evaluating Mitigation Options in Smallholder Agriculture. Springer, Cham. https://doi.org/10.1007/978-3-319-29794-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-29794-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-29792-7
Online ISBN: 978-3-319-29794-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)