Abstract
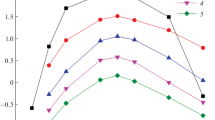
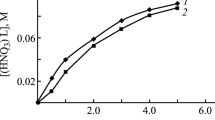
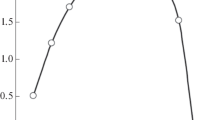
Extraction of lanthanides and actinides from nitric acid solutions with N-(diphenylphosphoryl)-N'-n-propylureas containing imidazolyl, diethylamino, pyrid-2-yl, 2-oxopyrrolidino fragments in the ω position of the alkyl substituent has been studied. It has been shown that Ho(III) and Yb(III) related to the yttrium subgroup of lanthanides are extracted much better than La(III) and Nd(III) related to the cerium subgroup. N-(Diphenylphosphoryl)urea containing ω-(2-oxopyrrolidino)propyl fragment at the terminal nitrogen atom shows the best extraction properties. This dependence has been theoretically explained by modeling complexation because the coordination of f-block element ion with amide oxygen atom is more preferable






Similar content being viewed by others
REFERENCES
A. M. Rozen and B. V. Krupnov, Russ. Chem. Rev. 65, 973 (1996). https://doi.org/10.1070/RC1996v065n11ABEH000241]
A. Leoncini, J. Huskens, and W. Verboom, Chem. Soc. Rev. 46, 7229 (2017). https://doi.org/10.1039/c7cs00574a
E. P. Horwitz, D. G. Kalina, L. Kaplan, et al., Sep. Sci. Technol. 17, 1261 (1982). https://doi.org/10.1080/01496398208060649
M. Jensen, R. Chiarizia, J. S. Ulicki, et al., Solvent Extr. Ion Exch. 33, 329 (2015). https://doi.org/10.1080/07366299.2015.1046292
A. T. Ta, G. A. Hegde, B. D. Etz, et al., J. Phys. Chem. B 122, 5999 (2018). https://doi.org/10.1021/acs.jpcb.8b03165
B. Mahanty, P. K. Mohapatra, A. Leoncini, et al., Sep. Purif. Technol. 229, 115846 (2019). https://doi.org/10.1016/j.seppur.2019.115846
P. K. Mohapatra, P. Kandwal, M. Iqbal, et al., Dalton Trans. 42, 4343 (2013). https://doi.org/10.1039/c3dt32967d
A. Sengupta, P. K. Mohapatra, P. Pathak, et al., New J. Chem. 41, 836 (2017). https://doi.org/10.1039/C6NJ03102A
J. C. Braley, G. J. Lumetta, J. C. Carter, et al., Solvent Extr. Ion Exch. 31, 567 (2013). https://doi.org/10.1080/07366299.2013.785912
Y. Sasaki and S. Umetani, J. Nucl. Sci. Technol. 43, 794 (2006). https://doi.org/10.1080/18811248.2006.9711161
E. V. Sharova, O. I. Artyushin, and I. L. Odinets, Russ. Chem. Rev. 83, 95 (2014). https://doi.org/10.1070/RC2014v083n02ABEH004384
A. N. Turanov, V. K. Karandashev, A. G. Matveeva, et al., Radiochemistry 59, 490 (2017). https://doi.org/10.1134/S1066362217050083
H. H. Dam, D. N. Reinhoudt, and W. Verboom, Chem. Soc. Rev. 36, 367 (2007). https://doi.org/10.1039/B603847F
D. A. Tatarinov, V. F. Mironov, A. A. Kostin, et al., Phosphorus, Sulfur Silicon Relat. Elem. 186, 694 (2011). https://doi.org/10.1080/10426507.2010.515955
N. E. Borisova, A. M. Safiullina, A. V. Lizunov, et al., Russ. J. Inorg. Chem. 64, 414 (2019). https://doi.org/10.1134/S0036023619030057
A. N. Turanov, V. K. Karandashev, V. E. Baulin, et al. Solvent Extr. Ion Exch. 27, 551 (2009). https://doi.org/10.1080/07366290903044683
A. N. Turanov, V. K. Karandashev, O. I. Artyushin, et al., Russ. J. Inorg. Chem. 65, 1226 (2020). https://doi.org/10.1134/S0036023620080185
S. V. Demin, S. E. Nefedov, V. I. Zhilov, et al., Russ. J. Inorg. Chem. 57, 897 (2012). https://doi.org/10.1134/S0036023612060095
A. N. Turanov, V. K. Karandashev, O. I. Artyushin, et al., Russ. J. Inorg. Chem. 65, 905 (2020). https://doi.org/10.31857/S0044457X20060240
I. G. Tananaev, A. A. Letyushov, A. M. Safiulina, et al., Dokl. Chem. 422, 260 (2008). https://doi.org/10.1134/S0012500808100054
E. I. Goryunov, A. E. Shipov, I. B. Goryunova, et al., Dokl. Chem. 438, 151 (2011). https://doi.org/10.1134/S0012500811060012
A. M. Safiulina, E. I. Goryunov, A. A. Letyushov, et al., Mendeleev Commun. 19, 263 (2009). https://doi.org/10.1016/j.mencom.2009.09.010
E. I. Goryunov, T. V. Baulina, I. B. Goryunova, et al., Russ. Chem. Bull. 63, 141 (2014). https://doi.org/10.1007/s11172-014-0408-y
P. S. Lemport, E. I. Goryunov, I. B. Goryunova, et al., Dokl. Chem. 425, 84 (2009). https://doi.org/10.1134/S0012500809040053
A. M. Safiulina, M. S. Grigoriev, E. E. Nifant’ev, et al., Russ. J. Inorg. Chem. 57, 108 (2012). https://doi.org/10.1134/S0036023612010196
S. B. Savvin, Organic Reagents of Arsenazo III Group (Atomizdat, Moscow, 1971).
D. N. Laikov, Chem. Phys. Lett. 416, 116 (2005). https://doi.org/10.1016/j.cplett.2005.09.046
D. N. Laikov, Chem. Phys. Lett. 281, 151 (1997). https://doi.org/10.1016/S0009-2614(97)01206-2
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett. 77, 3865 (1996). https://doi.org/10.1103/PhysRevLett.77.3865
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett. 78, 1396 (1997). https://doi.org/10.1103/PhysRevLett.78.1396
N. E. Borisova, A. A. Kostin, E. A. Eroshkina, et al., Eur. J. Inorg. Chem. 13, 2219 (2014). https://doi.org/10.1002/ejic.201301271
ACKNOWLEDGMENTS
This work was supported by the Ministry of Science and Higher Education of the Russian Federation using equipment of the Center for Molecular Structure Studies, Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences.
The computations were performed on a MVS-50K Supercomputer of the Joint Supercomputer Center, Russian Academy of Sciences (www.jscc.ru).
Funding
Computations were supported by the Russian Science Foundation (project no. 16–13–10451).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
In memory of eminent Soviet and Russian organophosphorus chemist E. E. Nifant’ev, Corresponding Member of the RAS
Translated by I. Kudryavtsev
Rights and permissions
About this article
Cite this article
Safiulina, A.M., Lizunov, A.V., Borisova, N.E. et al. Extraction Properties of Diphenylposphorylureas with Aliphatic ω-Nitrogen-Containing Substituents. Russ. J. Inorg. Chem. 66, 731–739 (2021). https://doi.org/10.1134/S0036023621050156
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621050156