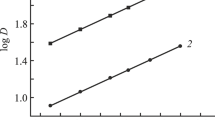
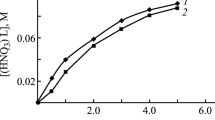
Abstract
The interphase distribution of La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu between aqueous HNO3 solutions and solutions of tetraphenylmethylenediphosphine dioxide in 1,2-dichloroethane was studied. The latter solutions also contained ionic liquids with an anion of bis[(trifluoromethyl)sulfonyl]imide and cations of quaternary ammonium bases. It was detected that the extraction of metal ions significantly increases in the presence of ionic liquids in the organic phase. The stoichiometry of the extracted complexes was determined, and analysis was made of the effect of the HNO3 concentration in the aqueous phase and the nature of the cationic part of an ionic liquid on the efficiency of the extraction of metal ions into the organic phase.






Similar content being viewed by others
REFERENCES
B. F. Myasoedov, S. N. Kalmykov, Yu. M. Kulyako, et al., Geochem. Int. 54, 1156 (2016). https://doi.org/10.1134/S0016702916130115
M. Yu. Alyapychev, V. A. Babain, and Yu. A. Ustynyuk, Russ. Chem. Rev. 85, 943 (2016). https://doi.org/10.1070/RCR4588
A. Leoncini, J. Huskens, and W. Verboom, Chem. Soc. Rev. 46, 7229 (2017). https://doi.org/10.1039/C7CS00574A
A. M. Wilson, P. J. Bailey, and P. A. Tasker, Chem. Soc. Rev. 43, 123 (2014). https://doi.org/10.1039/C3CS60275C
A. M. Rozen, V. I. Volk, A. Yu. Vakhrushin, Radiochemistry 41, 215 (1999).
A. M. Rozen and B. V. Krupnov, Russ. Chem. Rev. 65, 973 (1996). https://doi.org/10.1070/RC1996v065n11ABEH000241
A. M. Rozen, Z. I. Nikolotova, N. A. Kartasheva, and K. S. Yudina, Dokl. Akad. Nauk SSSR 222, 1151 (1975).
A. E. Visser, R. P. Swatloski, W. M. Reichert, et al., Ind. Eng. Chem. Res. 39, 3596 (2000).
M. Koel, CRC Crit. Rev. Anal. Chem. 35, 177 (2005). https://doi.org/10.1080/10408340500304016
H. Zhao, S. Xia, and P. Ma, J. Chem. Technol. Biotechnol. 80, 1089 (2005).
M. L. Dietz, Sep. Sci. Technol. 41, 2047 (2006).
K. Nakashima, F. Kubota, T. Maruyama, et al., Anal. Sci. 19, 1097 (2003). https://doi.org/10.2116/analsci.19.1097
A. E. Visser and R. D. Rogers, J. Solid State Chem. 171, 109 (2003).
Z. Kolarik, Solvent Extr. Ion Exch. 31, 24 (2013). https://doi.org/10.1080/07366299.2012.700589
F. Kubota, Y. Baba, and M. Goto, Solvent Extr. Res. Dev. Jpn. 19, 17 (2012).
I. Billard, in Handbook on the Physics and Chemistry of Rare Earths, Ed. by J.-C. G. Bünzli and V. Pecharsky (Elsevier, Amsterdam, 2013), Vol. 43, Ch. 256, p. 213.
I. A. Shkrob, T. W. Marin, and M. P. Jensen, Ind. Eng. Chem. Res. 53, 3641 (2014). https://doi.org/10.1021/ie4036719
P. K. Mohapatra, Chem. Prod. Proc. Model. 10, 135 (2015).
N. K. Gupta, J. Mol. Liq. 269, 72 (2018).
C. A. Hawkins, M. A. Momen, and M. L. Dietz, Sep. Sci. Technol. 53, 1820 (2018). https://doi.org/1080/01496395.2017.1302478
A. N. Turanov, V. K. Karandashev, and V. E. Baulin, Russ. J. Inorg. Chem. 53, 970 (2008). https://doi.org/10.1134/S0036023608060272
A. N. Turanov, V. K. Karandashev, and V. E. Baulin, Radiochemistry 50, 266 (2008). https://doi.org/10.1134/S1066362208030090
A. N. Turanov, V. K. Karandashev, and A. N. Yarkevich, Radiochemistry 55, 382 (2013).
A. N. Turanov, V. K. Karandashev, and A. N. Yarkevich, Russ. J. Inorg. Chem. 63, 406 (2018). https://doi.org/10.1134/S0036023618030221
T. J. Bell and Y. Ikeda, Dalton Trans. 40, 10125 (2011).
V. A. Chauzov, Yu. N. Studnev, M. G. Iznostkova, and A. V. Fokin, Zh. Obshch. Khim. 57, 54 (1987).
P. Bonhote, A. P. Dias, N. Papageorgiou, et al., Inorg. Chem. 35, 1168 (1996).
H. Naganawa, H. Suzuki, S. Tachimori, et al., Phys. Chem. Chem. Phys. 3, 2509 (2001).
H. Suzuki, H. Naganawa, and S. Tachimori, Phys. Chem. Chem. Phys. 5, 726 (2003).
J. Rais and S. Tachimori, J. Radioanal. Nucl. Chem. Lett. 188, 157 (1994).
S. Dai, Y. H. Ju, and C. E. Barnes, J. Chem. Soc., Dalton Trans. 1201 (1999).
K. B. Yatsimirskii, N. A. Kostromina, Z. A. Sheka, et al., Chemistry of Complex Compounds of Rare-Earth Elements (Nauk. Dumka, Kiev, 1966) [in Russian].
A. N. Turanov, V. K. Karandashev, and V. E. Baulin, Solvent Extr. Ion Exch. 26, 77 (2008). https://doi.org/10.1080/07366290801904871
A. N. Turanov, V. K. Karandashev, and V. A. Khvostikov, Solvent Extr. Ion Exch. 35, 461 (2017).
C. Gaillard, M. Boltoeva, I. Billard, et al., Chem. Phys. Chem. 16, 2653 (2015). https://doi.org/10.1002/cphc.201500283
A. N. Turanov, V. K. Karandashev, and V. E. Baulin, Radiochemistry 43, 72 (2001).
K. Binnemans, Chem. Rev. 107, 2593 (2007). https://doi.org/10.1021/cr050979c
Funding
This work was performed under the 2019 state assignment for the Institute of Solid-State Physics, Russian Academy of Sciences, and the Institute of Problems of Microelectronics Technology and High-Purity Materials, Russian Academy of Sciences (both in Chernogolovka, Moscow oblast, Russia).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Turanov, A.N., Karandashev, V.K. Extraction of Lanthanides(III) from Nitric Acid Solutions with Tetraphenylmethylenediphosphine in the Presence of Bis[(trifluoromethyl)sulfonyl]imides of Quaternary Ammonium Bases. Russ. J. Inorg. Chem. 65, 113–118 (2020). https://doi.org/10.1134/S0036023620010180
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620010180