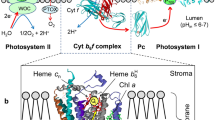
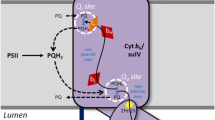
Abstract
This work represents an overview of electron transport regulation in chloroplasts as considered in the context of structure-function organization of photosynthetic apparatus in plants. Main focus of the article is on bifurcated oxidation of plastoquinol by the cytochrome b6f complex, which represents the rate-limiting step of electron transfer between photosystems II and I. Electron transport along the chains of non-cyclic, cyclic, and pseudocyclic electron flow, their relationships to generation of the trans-thylakoid difference in electrochemical potentials of protons in chloroplasts, and pH-dependent mechanisms of regulation of the cytochrome b6f complex are considered. Redox reactions with participation of molecular oxygen and ascorbate, alternative mediators of electron transport in chloroplasts, have also been discussed.





Similar content being viewed by others
Abbreviations
- Asc, MDHA, DHA:
-
three redox forms of ascorbate (fully reduced, semiquinone, and completely oxidized)
- CBC:
-
Calvin–Benson cycle
- CET:
-
cyclic electron transport
- EPR:
-
electron paramagnetic resonance
- ETC:
-
electron transport chain
- ISP:
-
iron-sulfur protein, part of PSI
- Fd:
-
ferredoxin
- FNR:
-
ferredoxin-NADP reductase
- NDH-1:
-
NAD(P)H-dehydrogenase of chloroplasts type 1
- P700 and P680 :
-
primary electron donors in PSI and PSII
- Pc:
-
plastocyanin
- PGR5 and PGRL1:
-
proteins involved in cyclic electron transfer around PSI
- PSI and PSII:
-
photosystem I and photosystem II
- PQ:
-
plastoquinone
- PQH2 :
-
plastoquinol
- PTOX:
-
plastid (plastoquinol) terminal oxidase
- ROS:
-
reactive oxygen species
References
Edwards, G., and Walker, D. (1983) C3, C4: Mechanisms, and Cellular and Environmental Regulation of Photosynthesis, Univ. of California Press, Berkeley.
Gurrieri, L., Fermani, S., Zaffagnini, M., Sparla, F., and Trost, P. (2021) Calvin–Benson cycle regulation is getting complex, Trends Plant Sci., 26, 898-912, https://doi.org/10.1016/j.tplants.2021.03.008.
Boyer, P. D. (1997) The ATP synthase – a splendid molecular machine, Annu. Rev. Biochem., 66, 717-749, https://doi.org/10.1146/annurev.biochem.66.1.717.
Junge, W., and Nelson, N. (2015) ATP synthase, Annu. Rev. Biochem., 83, 631-657, https://doi.org/10.1146/annurev-biochem-060614-034124.
Romanovsky, Y. M., and Tikhonov, A. N. (2010) Molecular energy transducers of the living cell. Proton ATP synthase: a rotating molecular motor, Physics Usp., 53, 893-914, https://doi.org/10.3367/UFNe.0180.201009b.0931.
Nelson, N., and Yocum, C. F. (2006) Structure and function of photosystems I and II, Annu. Rev. Plant Biol., 57, 521-565, https://doi.org/10.1146/annurev.arplant.57.032905.105350.
Mamedov, M., Govindjee, Nadtochenko, V., and Semenov, A. Yu. (2015) Primary electron transfer processes in photosynthetic reaction centers from oxygenic organisms, Photosynth. Res., 125, 51-63, https://doi.org/10.1007/s11120-015-0088-y.
Brettel, K. (1997) Electron transfer and arrangement of the redox cofactors in photosystem I, Biochim. Biophys. Acta, 1318, 322-373, https://doi.org/10.1016/S0005-2728(96)00112-0.
Allakhverdiev, S. I. (2011) Recent progress in the studies of structure and function of photosystem II, J. Photochem. Photobiol. B, 104, 1-8, https://doi.org/10.1016/j.jphotobiol.2011.03.010.
Müh, F., Glöckner, C., Hellmich, J., and Zouni, A. (2012) Light-induced quinone reduction in photosystem II, Biochim. Biophys. Acta, 1817, 44-65, https://doi.org/10.1016/j.bbabio.2011.05.021.
Shevela, D., Kern, J. F., Govindjee, G., and Messinger, J. (2023) Solar energy conversion by photosystem II: principles and structures, Photosynth. Res., 156, 279-307, https://doi.org/10.1007/s11120-022-00991-y.
Tikhonov, A. N. (2014) The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways, Plant Physiol. Biochem., 81, 163-183, https://doi.org/10.1016/j.plaphy.2013.12.011.
Cramer, W. A., and Hasan, S. S. (2016) Structure-function of the cytochrome b6f lipoprotein complex, in Cytochrome Complexes: Evolution, Structures, Energy Transduction, and Signaling (Cramer, W. A., and Kallas, T., eds) Springer, Dordrecht, pp. 177-207, https://doi.org/10.1007/978-94-017-7481-9_9.
Tikhonov, A. N. (2018) The cytochrome b6f complex: biophysical aspects of its functioning in chloroplasts, in Membrane Protein Complexes: Structure and Function, Subcellular Biochemistry (Harris, J. R., Boekema, E. J., eds) 87, Springer Nature, Singapore Pte Ltd., pp. 287-328, https://doi.org/10.1007/978-981-10-7757-9_10.
Malone, L. A., Proctor, M. S., Hitchcock, A., Hunter, C. N., and Johnson, M. P. (2021) Cytochrome b6f – orchestrator of photosynthetic electron transfer, Biochim. Biophis. Acta, 1862, 148380, https://doi.org/10.1016/j.bbabio.2021.148380.
Sarewicz, M., Pintscher, S., Pietras, R., Borek, A., Bujnowicz, Ł., Hanke, G., Cramer, W. A., Finazzi, G., and Osyczka, A. (2021) Catalytic reactions and energy conservation in the cytochrome bc1 and b6f complexes of energy-transducing membranes, Chem. Rev., 121, 2020-2108, https://doi.org/10.1021/acs.chemrev.0c00712.
Sarewicz, M., Szwalec, M., Pintscher, S., Indyka, P., Rawski, M., Pietras, R., Mielecki, B., Koziej, Ł., Jaciuk, M., Glatt, S., and Osyczka, A. (2023) High-resolution cryo-EM structures of plant cytochrome b6f at work, Sci. Adv., 9, 1-12, https://doi.org/10.1126/sciadv.add9688.
Staehelin, L. A. (2003) Chloroplast structure: from chlorophyll granules to supramolecular architecture of thylakoid membranes, Photosynth. Res., 76, 185-196, https://doi.org/10.1023/A:1024994525586.
Albertsson, P.-Å. (2001) A quantitative model of the domain structure of the photosynthetic membrane, Trends Plant Sci., 6, 349-354, https://doi.org/10.1016/S1360-1385(01)02021-0.
Dekker, J. P., and Boekema, E. J. (2005) Supramolecular organization of thylakoid membrane proteins in green plants, Biochim. Biophys. Acta, 1706, 12-39, https://doi.org/10.1016/j.bbabio.2004.09.009.
Pribil, M., Labs, M., and Leister, D. (2014) Structure and dynamics of thylakoids in land plants, Environ. Bot., 65, 1955-1972, https://doi.org/10.1093/jxb/eru090.
Kramer, D. M., Avenson, T. J., and Edwards, G. E. (2004) Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions, Trends Plant. Sci., 9, 349-357, https://doi.org/10.1016/j.tplants.2004.05.001.
Johnson, M. P., and Ruban, A. V. (2014) Rethinking the existence of a steady-state Δψ component of the proton motive force across plant thylakoid membranes, Photosynth. Res., 119, 233-242, https://doi.org/10.1007/s11120-013-9817-2.
Bendall, D. S., and Manasse, R. S. (1995) Cyclic photophosphorylation and electron transport, Biochim. Biophys. Acta, 1229, 23-38, https://doi.org/10.1016/0005-2728(94)00195-B.
Joliot, P., and Joliot, A. (2006) Cyclic electron flow in C3 plants, Biochim. Biophys. Acta, 1757, 362-368, https://doi.org/10.1016/j.bbabio.2006.02.018.
Joliot, P., Sellés, J., Wollman, F.-A., and Verméglio, A. (2022) High efficient cyclic electron flow and functional supercomplexes in Chlamydomonas cells, Biochim. Biophys. Acta Bioenerg., 1863, 148909, https://doi.org/10.1016/j.bbabio.2022.148909.
Iwai, M., Takizawa, K., Tokutsu, R., Okamuro, A., Takahashi, Y., and Minagawa, J. (2010) Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis, Nature, 464, 1210-1213, https://doi.org/10.1038/nature08885.
Munekage, Y., Hojo, M., Meurer, J., Endo, T., Tasaka, M., and Shikanai, T. (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis, Cell, 110, 361-371, https://doi.org/10.1016/S0092-8674(02)00867-X.
DalCorso, G., Pesaresi, P., Masiero, S., Aseeva, E., Schünemann, D., Finazzi, G., Joliot, P., Barbato, R., and Leister, D. (2008) A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis, Cell, 132, 273-285, https://doi.org/10.1016/j.cell.2007.12.028.
Buchert, F., Mosebach, L., Gäbelein, P., and Hippler, M. (2020) PGR5 is required for efficient Q cycle in the cytochrome b6f complex during cyclic electron flow, Biochem. J., 477, 1631-1650, https://doi.org/10.1042/BCJ20190914.
Strand, D. D., Fisher, N., and Kramer, D. M. (2016) Distinct energetics and regulatory functions of the two major cyclic electron flow pathways in chloroplasts, in Chloroplasts: Current Research and Future Trends (Kirchhoff, H., ed.) Norfolk, UK, Caister Academic Press, pp. 89-100, https://doi.org/10.21775/9781910190470.04.
Shikanai, T. (2016) Chloroplast NDH: A different enzyme with a structure similar to that of respiratory NADH dehydrogenase, Biochim. Biophys. Acta, 1857, 1015-1022, https://doi.org/10.1016/j.bbabio.2015.10.013.
Laughlin, T. G., Bayne, A. N., Trempe, J. F., Savage, D. F., and Davies, K. M. (2019) Structure of the complex I-like molecule NDH of oxygenic photosynthesis, Nature, 566, 411-414, https://doi.org/10.1038/s41586-019-0921-0.
Schuller, J. M., Birrell, J. A., Tanaka, H., Konuma, T., Wulfhorst, H., Cox, N., Schuller, S. K., Thiemann, J., Lubitz, W., Sétif, P., Ikegami, T., Engel, B. D., Kurisu, G., and Nowaczyk, M. M. (2019) Structural adaptations of photosynthetic complex I enable ferredoxin-dependent electron transfer, Science, 363, 257-260, https://doi.org/10.1126/science.aau3613.
Zhang, C., Shuai, J., Ran, Z., Zhao, J., Wu, Z., Liao, R., Wu, J., Ma, W., and Lei, M. (2020) Structural insights into NDH-1 mediated cyclic electron transfer, Nat. Commun., 11, 888, https://doi.org/10.1038/s41467-020-14732-z.
Stiehl, H. H., and Witt, H. T. (1969) Quantitative treatment of the function of plastoquinone in photosynthesis, Z. Naturforsch. B, 24, 1588-1598, https://doi.org/10.1515/znb-1969-1219.
Haehnel, W. (1976) The reduction kinetics chlorophyll aI as indicator for proton uptake between the light reactions in chloroplasts, Biochim. Biophys. Acta, 440, 506-521, https://doi.org/10.1016/0005-2728(76)90038-4.
Tikhonov, A. N., Khomutov, G. B., and Ruuge, E. K. (1984) Electron transport control in chloroplasts. Effects of magnesium ions on the electron flow between two photosystems, Photobiochem. Photobiophys., 8, 261-269.
Haehnel, W. (1984) Photosynthetic electron transport in higher plants, Annu. Rev. Plant Physiol., 35, 659-693, https://doi.org/10.1146/annurev.pp.35.060184.003303.
Höhner, R., Pribil, M., Herbstová, M., Lopez, L. S., Kunz, H.-H., Li, M., Wood, M., Svoboda, M., Puthiyaveetil, S., Leister, L., and Kirchhoff, H. (2020) Plastocyanin is the long-range electron carrier between photosystem II and photosystem I in plants, Proc. Natl. Acad. Sci. USA, 117, 15354-15362, https://doi.org/10.1073/pnas.2005832117.
Tikhonov, A. N. (2013) pH-Dependent regulation of electron transport and ATP synthesis in chloroplasts, Photosynth. Res., 116, 511-534, https://doi.org/10.1007/s11120-013-9845-y.
Berry, E. A., Guergova-Kuras, M., Huang, L. S., and Crofts, A. R. (2000) Structure and function of cytochrome bc complexes, Annu. Rev. Biochem., 69, 1005-1075, https://doi.org/10.1146/annurev.biochem.69.1.1005.
Mitchell, P. (1976) Possible molecular mechanisms of the protonmotive function of cytochrome systems, J. Theor. Biol., 62, 327-367, https://doi.org/10.1016/0022-5193(76)90124-7.
Cramer, W. A., Hasan, S. S., and Yamashita, E. (2011) The Q cycle of cytochrome bc complexes: a structure perspective, Biochim. Biophys. Acta, 1807, 788-802, https://doi.org/10.1016/j.bbabio.2011.02.006.
Chow, W. S., and Hope, A. B. (2004) Kinetics of reactions around the cytochrome bf complex studied in intact leaf disks, Photosynth. Res., 81, 153-163, https://doi.org/10.1023/B:PRES.0000035027.02655.8c.
Ivanov, B. (1993) Stoichiometry of proton uptake by thylakoids during electron transport in chloroplasts, in Photosynthesis: Photoreactions to Plant Productivity, Springer, Dordrecht, (Abrol, Y. P., Mohanty, P., and Govindjee, G., eds) pp. 108-128, https://doi.org/10.1007/978-94-011-2708-0_4.
Kirchhoff, H., Hall, C., Wood, M., Herbstová, M., Tsabari, O., Nevo, R., Charuvi, D., Shimoni, E., and Reich, Z. (2011) Dynamic control of protein diffusion within the granal thylakoid lumen, Proc. Natl. Acad. Sci. USA, 108, 20248-20253, https://doi.org/10.1073/pnas.1104141109.
Mehler, A. H. (1951) Studies on reactions of illuminated chloroplasts: I. Mechanism of the reduction of oxygen and other hill reagents, Arch. Biochem. Biophys., 33, 65-77, https://doi.org/10.1016/0003-9861(51)90082-3.
Asada, K. (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons, Ann. Rev. Plant Physiol. Plant Mol. Biol., 50, 601-639, https://doi.org/10.1146/annurev.arplant.50.1.601.
Heber, U. (2002) Irrungen, Wirrungen? The Mehler reaction in relation to cyclic electron transport in C3 plants, Photosynth. Res., 73, 223-231, https://doi.org/10.1023/A:1020459416987.
Ivanov, B., Khorobryh, S., Kozuleva, M., and Borisova-Mubarakhshina, M. (2014) The Role of Oxygen and Its Reactive Forms in Photosynthesis [in Russian], Sovremennyie Problemy Fotosinteza (Allakhverdiev, S. I., Rubin, A. B., and Shuvalov, V. A., eds) Izhevsk Institute of Computer Research, Izhevsk-Moscow, 1, 407-460.
Nixon, P. J. (2000) Chlororespiration, Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci., 355, 1541-1547, https://doi.org/10.1098/rstb.2000.0714.
Peltier, G., and Cournac, L. (2002) Chlororespiration, Annu. Rev. Plant Biol., 53, 523-550, https://doi.org/10.1146/annurev.arplant.53.100301.13524.
Joët, T., Genty, B., Josse, E.-M., Kuntz, M., Cournac, L., and Peltier, G. (2002) Involvement of a plastid terminal oxidase in plastoquinone oxidation as evidenced by expression of the Arabidopsis thaliana enzyme in tobacco, J. Biol. Chem., 277, 31623-31630, https://doi.org/10.1074/jbc.M203538200.
McDonald, A. E., Ivanov, A. G., Bode, R., Maxwell, D. P., Rodermel, S. R., and Huner, N. P. A. (2011) Flexibility in photosynthetic electron transport: The physiological role of plastoquinol terminal oxidase (PTOX), Biochim. Biophys. Acta, 1807, 954-967, https://doi.org/10.1016/j.bbabio.2010.10.024.
Nawrocki, W. J., Tourasse, N. J., Taly, A., Rappaport, F., and Wollman, F.-A. (2015) The plastid terminal oxidase: its elusive function points to multiple contributions to plastid physiology, Annu. Rev. Plant Biol., 66, 49-74, https://doi.org/10.1146/annurev-arplant-043014-114744.
Lennon, A. M., Prommeenate, P., and Nixon, P. J. (2003) Location, expression and orientation of the putative chlororespiratory enzymes, Ndh and IMMUTANS, in higher-plant plastids, Planta, 218, 254-260, https://doi.org/10.1007/s00425-003-1111-7.
Ifuku, K., Endo, T., Shikanai, T., and Aro, E.-M. (2011) Structure of the chloroplast NADH dehydrogenase-like complex: nomenclature for nuclear-encoded subunits, Plant Cell Physiol., 52, 1560-1568, https://doi.org/10.1093/pcp/pcr098.
Foyer, C. H., and Noctor, G. (2011) Ascorbate and glutathione: the heart of the redox hub, Plant Physiol., 155, 2-18, https://doi.org/10.1104/pp.110.167569.
Anderson, J. M. (1982) Distribution of the cytochromes of spinach chloroplasts between the appressed membranes of grana stacks and stroma-exposed thylakoid regions, FEBS Lett., 138, 62-66, https://doi.org/10.1016/0014-5793(82)80395-5.
Vallon, O., Bulte, L., Dainese, P., Olive, J., Bassi, R., and Wollman, F.-A. (1991) Lateral redistribution of cytochrome b6/f complexes along thylakoid membranes upon state transitions, Proc. Natl. Acad. Sci. USA, 88, 8262-8266, https://doi.org/10.1073/pnas.88.18.8262.
Dumas, L., Chazaux, M., Peltier, G., Johnson, X., and Alric, J. (2016) Cytochrome b6f function and localization, phosphorylation state of thylakoid membrane proteins and consequences on cyclic electron flow, Photosynth. Res., 129, 307-320, https://doi.org/10.1007/s11120-016-0298-y.
Kirchhoff, H., Li, M., and Puthiyaveetil, S. (2017) Sublocalization of cytochrome b6f complexes in photosynthetic membranes, Trends Plant. Sci., 22, 574-582, https://doi.org/10.1016/j.tplants.2017.04.004.
Kirchhoff, H., Horstmann, S., and Weis, E. (2000) Control of the photosynthetic electron transport by PQ diffusion in microdomains in thylakoids of higher plants, Biochim. Biophys. Acta, 1459, 148-168, https://doi.org/10.1016/S0005-2728(00)00143-2.
Wood, W. H. J., MacGregor-Chatwin, C., Barnett, S. F. H., Mayneord, G. E., Huang, X., Hobbs, J. K., Hunter, C. N., and Johnson, M. P. (2018) Dynamic thylakoid stacking regulates the balance between linear and cyclic photosynthetic electron transfer, Nat. Plants, 4, 116-127, https://doi.org/10.1038/s41477-017-0092-7.
Hanke, G. T., Kimata-Ariga, Y., Taniguchi, I., and Hase, T. (2004) A post genomic characterization of Arabidopsis ferredoxins, Plant Physiol., 134, 255-264, https://doi.org/10.1104/pp.103.032755.
Hanke, G. T., and Hase, T. (2008) Variable photosynthetic roles of two leaf-type ferredoxins in Arabidopsis, as revealed by RNA interference, Photochem. Photobiol., 84, 1302-1309, https://doi.org/10.1111/j.1751-1097.2008.00411.x.
Blanco, N., Ceccoli, R., Dalla Via, M. V., Voss, I., Segretin, M. E., Bravo-Almonacid, F. F., Melzer, M., Hajirezaei, M.-R., Scheibe, R., and Hanke, G. T. (2013) Expression of the minor isoform pea ferredoxin in tobacco alters photosynthetic electron partitioning and enhances cyclic electron flow, Plant Physiol., 161, 866-879, https://doi.org/10.1104/pp.112.211078.
Lehtimäki, N., Lintala, M., Allahverdiyeva, Y., Aro, E. M., and Mulo, P. (2010) Drought stress-induced upregulation of components involved in ferredoxin-dependent cyclic electron transfer, J. Plant Physiol., 167, 1018-1022, https://doi.org/10.1016/j.jplph.2010.02.006.
Gins, V. K., Tikhonov, A. N., Mukhin, E. N., and Ruuge, E. K. (1982) Features of the functioning of two molecular forms of pea ferredoxin in the electron transport chain of chloroplasts [in Russian], Biokhimiya, 47, 1859-1866.
Gong, X.-S., Chung, S., and Fernandez-Velasco, J. G. (2001) Electron transfer and stability of the cytochrome b6f complex in a small domain deletion mutant of cytochrome f, J. Biol. Chem., 276, 24365-24371, https://doi.org/10.1074/jbc.M010721200.
Yan, J., and Cramer, W. A. (2003) Functional insensitivity of the cytochrome b6f complex to structure changes in the hinge region of the Rieske iron-sulfur protein, J. Biol. Chem., 278, 20925-20933, https://doi.org/10.1074/jbc.M212616200.
Rumberg, B., and Siggel, U. (1969) pH changes in the inner phase of the thylakoids during photosynthesis, Naturwissenschaften, 56, 130-132, https://doi.org/10.1007/BF00601025.
Tikhonov, A. N., Khomutov, G. B., Ruuge, E. K., and Blumenfeld, L. A. (1981) Electron transport control in chloroplasts. Effects of photosynthetic control monitored by the intrathylakoid pH, Biochem. Biophys. Acta, 637, 321-333, https://doi.org/10.1016/0005-2728(81)90171-7.
Kramer, D. M., Sacksteder, C. A., and Cruz, J. A. (1999) How acidic is the lumen? Photosynth. Res., 60, 151-163, https://doi.org/10.1023/A:1006212014787.
Tikhonov, A. N. (2012) Energetic and regulatory role of proton potential in chloroplasts, Biochemistry (Moscow), 77, 956-974, https://doi.org/10.1134/S0006297912090027.
Li, Z., Wakao, S., Fischer, B. B., and Niyogi, K. K. (2009) Sensing and responding to excess light, Annu. Rev. Plant Biol., 60, 239-260, https://doi.org/10.1146/annurev.arplant.58.032806.103844.
Demmig-Adams, B., Cohu, C. M., Muller, O., and Adams, W. W. (2012) Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons, Photosynth. Res., 113, 75-88, https://doi.org/10.1007/s11120-012-9761-6.
Horton, P. (2012) Optimization of light harvesting and photoprotection: molecular mechanisms and physiological consequences, Philos. Trans. R. Soc. Lond. B Biol. Sci., 367, 3455-3465, https://doi.org/10.1098/rstb.2012.0069.
Brandt, U. (1996) Bifurcated ubihydroquinone oxidation in the cytochrome bc1 complex by proton-gated charge transfer, FEBS Lett., 387, 1-6, https://doi.org/10.1016/0014-5793(96)00436-X.
Link, T. A. (1997) The role of the “Rieske” iron sulfur protein in the hydroquinone oxidation (Qp) site of the cytochrome bc1 complex: the “proton-gated affinity change” mechanism, FEBS Lett., 412, 257-264, https://doi.org/10.1016/S0014-5793(97)00772-2.
Zu, Y., Manon, M.-J., Couture, M. M.-J., Kolling, D. R. J., Crofts, A. R., Eltis, L. D., Fee, J. A., and Hirst, J. (2003) The reduction potentials of Rieske clusters: the importance of the coupling between coupling between oxidation state and histidine protonation state, Biochemistry, 42, 12400-12408, https://doi.org/10.1021/bi0350957.
Crofts, A. R., Guergova-Kuras, M., Kuras, R., Ugulava, N., Li, J., and Hong, S. (2000) Proton-coupled electron transfer at the Qo site: What type of mechanism can account for the high activation barrier? Biochim. Biophys. Acta, 1459, 456-466, https://doi.org/10.1016/S0005-2728(00)00184-5.
Zhu, J., Egawa, T., Yeh, S.-R., Yu, L., and Yu, C.-A. (2007) Simultaneous reduction of iron–sulfur protein and cytochrome bL during ubiquinol oxidation in cytochrome bc1 complex, Proc. Natl. Acad, Sci. USA, 104, 4864-4869, https://doi.org/10.1073/pnas.0607812104.
Osyczka, A., Moser, C. C., and Dutton, P. L. (2005) Fixing the Q cycle, Trends. Biochem. Sci., 30, 176-182, https://doi.org/10.1016/j.tibs.2005.02.001.
Ustynyuk, L. Yu., and Tikhonov, A. N. (2018) The cytochrome b6f complex: DFT modeling of the first step of plastoquinol oxidation by the iron-sulfur protein, J. Organomet. Chem., 867, 290-299, https://doi.org/10.1016/j.jorganchem.2018.01.023.
Ustynyuk, L. Yu., and Tikhonov, A. N. (2022) Plastoquinol oxidation: rate-limiting stage in the electron transport chain of chloroplasts, Biochemistry (Moscow), 87, 1084-1097, https://doi.org/10.1134/S0006297922100029.
Postila, P. A., Kaszuba, K., Sarewicz, M., Osyczka, A., Vattulainen, I., and Róg, T. (2013) Key role of water in proton transfer at the Qo-site of the cytochrome bc1 complex predicted by atomistic molecular dynamics simulations, Biochim. Biophys. Acta, 1827, 761-768, https://doi.org/10.1016/j.bbabio.2013.02.005.
Chance, B., and Williams, G. R. (1956) The respiratory chain and oxidative phosphorylation, Adv. Enzymol., 17, 65-134, https://doi.org/10.1002/9780470122624.ch2.
Foyer, C. H., Neukermans, J., Queval, G., Noctor, G., and Harbinson, J. (2012) Photosynthetic control of electron transport and the regulation of gene expression, J. Exp. Bot., 63, 1637-1661, https://doi.org/10.1093/jxb/ers013.
Tikhonov, A. N., Agafonov, R. V., Grigor’ev, I. A., Kirilyuk, I. A., Ptushenko, V. V., and Trubitsin, B. V. (2008) Spin-probes designed for measuring the intrathylakoid pH in chloroplasts, Biochim. Biophys. Acta, 1777, 285-294, https://doi.org/10.1016/j.bbabio.2007.12.002.
Vershubskii, A. V., Trubitsin, B. V., Priklonskii, V. I., and Tikhonov, A. N. (2017) Lateral heterogeneity of the proton potential along the thylakoid membranes of chloroplasts, Biochim. Biophys. Acta, 1859, 388-401, https://doi.org/10.1016/j.bbamem.2016.11.016.
Buchanan, B. B. (1984) The ferredoxin/thioredoxin system: a key element in the regulatory function of light in photosynthesis, Bioscience, 34, 378-383, https://doi.org/10.2307/1309730.
Dietz, K. J. (2003) Redox control, redox signaling and redox homeostasis in plant cells, Int. Rev. Cytol., 228, 141-193, https://doi.org/10.1016/S0074-7696(03)28004-9.
Selinski, J., and Scheibe, R. (2019) Malate valves: old shuttles with new perspectives, Plant Biol., 21 (Suppl. 1), 21-30, https://doi.org/10.1111/plb12869.
Scheibe, R. (2019) Maintaining homeostasis by controlled alternatives for energy distribution in plant cells under changing conditions of supply and demand, Photosynth. Res., 139, 81-91, https://doi.org/10.1007/s11120-018-0583-z.
Tikhonov, A. N. (2015) Induction events and short-term regulation of electron transport in chloroplasts: An overview, Photosynth Res., 125, 65-94, https://doi.org/10.1007/s11120-015-0094-0.
Trubitsin, B. V., Vershubskii, A. V., Priklonskii, V. I., and Tikhonov, A. N. (2015) Short-term regulation and alternative pathways of photosynthetic electron transport in Hibiscus rosa-sinensis leaves, J. Photochem. Photobiol. B, 152, 400-415, https://doi.org/10.1016/j.jphotobiol.2015.07.015.
Kozuleva, M. A., Petrova, A. A., Mamedov, M. D., Semenov, A. Y., and Ivanov, B. N. (2014) O2 reduction by photosystem I involves phylloquinone under steady-state illumination, FEBS Lett., 588, 4364-4368, https://doi.org/10.1016/j.febslet.2014.10.003.
Kozuleva, M. A., and Ivanov, B. N. (2016) The mechanisms of oxygen reduction in the terminal reducing segment of the chloroplast photosynthetic electron transport chain, Plant Cell Physiol., 57, 1397-1404, https://doi.org/10.1093/pcp/pcw035.
Cherepanov, D. A., Milanovsky, G. E., Petrova, A. A., Tikhonov, A. N., Semenov, A. Yu. (2017) Electron transfer through the acceptor side of Photosystem I: Interaction with exogenous acceptors and molecular oxygen, Biochemistry (Moscow), 82, 1249-1268, https://doi.org/10.1134/S0006297917110037.
Kozuleva, M. A., and Ivanov, B. N. (2010) Evaluation of the participation of ferredoxin in oxygen reduction in the photosynthetic electron transport chain of isolated pea thylakoids, Photosynth. Res., 105, 51-61, https://doi.org/10.1007/s11120-010-9565-5.
Kuvykin, I. V., Vershubskii, A. V., Ptushenko, V. V., Tikhonov, A. N. (2008) Oxygen as an alternative electron acceptor in the photosynthetic electron transport chain of C3 plants, Biochemistry (Moscow), 73, 1063-1075, https://doi.org/10.1134/S0006297908100027.
Kuvykin, I. V., Ptushenko, V. V., Vershubskii, A. V., and Tikhonov, A. N. (2011) Regulation of electron transport in C3 plant chloroplasts in situ and in silico. Short-term effects of atmospheric CO2 and O2, Biochim. Biophys. Acta, 1807, 336-347, https://doi.org/10.1016/j.bbabio.2010.12.012.
Tikhonov, A. N., and Subczynski, W. K. (2019) Oxygenic photosynthesis: EPR study of photosynthetic electron transport and oxygen-exchange, an overview, Cell Biochem. Biophys., 77, 47-59, https://doi.org/10.1007/s12013-018-0861-6.
Trubitsin, B. V., Ptushenko, V. V., Koksharova, O. A., Mamedov, M. D., Vitukhnovskaya, L. A., Grigor’ev, I. A., Semenov, A. Yu., and Tikhonov, A. N. (2005) EPR study of electron transport in the cyanobacterium Synechocystis sp. PCC 6803: Oxygen-dependent interrelations between photosynthetic and respiratory electron transport chains, Biochim. Biophys. Acta, 1708, 238-249, https://doi.org/10.1016/j.bbabio.2005.03.004.
Baniulis, D., Hasan, S. S., Stofleth, J. T., and Cramer, W. A. (2013) Mechanism of enhanced superoxide production in the cytochrome b6f complex of oxygenic photosynthesis, Biochemistry, 52, 8975-8983, https://doi.org/10.1021/bi4013534.
Ivanov, B. N. (2008) Cooperation of photosystem I with the plastoquinone pool in oxygen reduction in higher plant chloroplasts, Biochemistry (Moscow), 73, 112-118, https://doi.org/10.1134/S0006297908010173.
Ivanov, B., Borisova-Mubarakshina, M., Vilyanen, D., Vetoshkina, D., Kozuleva, M. (2022) Cooperative pathway of O2 reduction to H2O2 in chloroplast thylakoid membrane: new insight into the Mehler reaction, Biophys. Rev., 14, 857-869, https://doi.org/10.1007/s12551-022-00980-4.
Ligeza, A., Tikhonov, A. N., Hyde, J. S., and Subczynski, W. K. (1998) Oxygen permeability of thylakoid membranes: electron paramagnetic resonance spin labeling study, Biochim. Biophys. Acta, 1365, 453-463, https://doi.org/10.1016/S0005-2728(98)00098-X.
Ligeza, A., Tikhonov, A. N., and Subczynski, W. K. (1997) In situ measurements of oxygen production and consumption using paramagnetic fusinite particles injected into a bean leaf, Biochim. Biophys. Acta, 1319, 133-137, https://doi.org/10.1016/S0005-2728(96)00122-3.
Rutherford, A. W., Osyczka, A., and Rappaport, F. (2012) Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: Redox tuning to survive life in O2, FEBS Lett., 586, 603-616, https://doi.org/10.1016/j.febslet.2011.12.039.
Foyer, C., Rowell, J., and Walker, D. (1983) Measurement of the ascorbate content of spinach leaves protoplasts and chloroplasts during illumination, Planta, 157, 239-244, https://doi.org/10.1007/BF00405188.
Law, M. Y., Charles, S. A., and Halliwell, B. (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat, Biochem. J., 210, 899-903, https://doi.org/10.1042/bj2100899.
Smirnoff, N. (1996) The function and metabolism of ascorbic acid in plants, Ann. Bot., 78, 661-669, https://doi.org/10.1006/anbo.1996.0175.
Gest, N., Gautier, H., and Stevens, R. (2013) Ascorbate as seen through plant evolution: the rise of a successful molecule? J. Exp. Bot., 64, 33-53, https://doi.org/10.1093/jxb/ers297.
Eskling, M., Arvidsson, P.-O., and Akerlund, H.-E. (1997) The xanthophylls cycle, its regulation and components, Physiol. Plant., 100, 806-816, https://doi.org/10.1034/j.1399-3054.1997.1000407.x.
Eskling, M., and Akerlund, H.-E. (1998) Changes in the quantities of violaxanthin deepoxidase, xanthophylls and ascorbate in spinach upon shift from low to high light, Photosynth. Res., 57, 41-50, https://doi.org/10.1023/A:1006015630167.
Müller-Moulé, P., Conclin, P. L., and Niyogy, K. K. (2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo, Plant Physiol., 128, 970-977, https://doi.org/10.1104/pp.010924.
Iyanagi, T., Yamazaki, I., and Anan, K. (1985) One-electron oxidation-reduction properties of ascorbic acid, Biochim. Biophys. Acta, 806, 255-261, https://doi.org/10.1016/0005-2728(85)90103-3.
Mano, J., Hideg, E., and Asada, K. (2004) Ascorbate in thylakoid lumen functions as an alternative electron donor to photosystem II and photosystem I, Arch. Biochim. Biophys., 429, 71-80, https://doi.org/10.1016/j.abb.2004.05.022.
Trubitsin, B. V., Mamedov, M. D., Semenov, A. Yu., and Tikhonov, A. N. (2014) Interaction of ascorbate with photosystem I, Photosynth. Res., 122, 215-231, https://doi.org/10.1007/s11120-014-0023-7.
Forti, G., and Ehrenheim, A. M. (1993) The role of ascorbic acid in photosynthetic electron transport, Biochim. Biophys. Acta, 1183, 408-412, https://doi.org/10.1016/0005-2728(93)90246-C.
Miyake, C., and Asada, K. (1994) Ferredoxin-dependent photoreduction of monodehydroascorbate radicals in spinach thylakoids, Plant Cell Physiol., 35, 539-549, https://doi.org/10.1093/oxfordjournals.pcp.a078628.
Mano, J., Ushimaru, T., and Asada, K. (1997) Ascorbate in thylakoids lumen as an endogenous electron donor to photosystem II: protection of thylakoids from photoinhibition and regeneration of ascorbate in stroma by dehydroascorbate reductase, Photosynth. Res., 53, 197-204, https://doi.org/10.1023/A:1005832022204.
Tóth, S. Z., Puthur, J. T., Nagi, V., and Garab, G. (2009) Experimental evidence for ascorbate-dependent electron transport in leaves with inactive oxygen evolving complexes, Plant Physiol., 149, 1568-1578, https://doi.org/10.1104/pp.108.132621.
Tóth, S. Z., Nagi, V., Puthur, J. T., Kovács, L., and Garab, G. (2011) The physiological role of ascorbate as photosystem II electron donor: protection against photoinactivation in heat-stressed leaves, Plant Physiol., 156, 382-392, https://doi.org/10.1104/pp.110.171918.
Acknowledgments
This article is dedicated to the memory of L. A. Drachev – one of the greatest Russian experimental biophysicists, whose scientific and technical developments and pioneering research in the field of photosynthesis contributed to a deeper understanding of the processes of charge separation and transfer in the reaction centers of photosynthetic systems.
The author is grateful to E. K. Ruuge, G. B. Khomutov, and L. Yu. Ustynyuk, together with whom the main results of the experimental and theoretical study of the regulation of electron transport in chloroplasts were previously obtained, to which the author refers in this review. The author thanks the anonymous reviewers for their helpful comments and recommendations.
Funding
This work was carried out within the framework of the research topic of the Faculty of Physics of Lomonosov Moscow State University “Physical Foundations of the Structure, Functioning and Regulation of Biological Systems” (State registration no. 012004 085 35) and with partial financial support from the Russian Foundation for Basic Research (grant no. 21-04-20047).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares no conflict of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by the author.
Rights and permissions
About this article
Cite this article
Tikhonov, A.N. Electron Transport in Chloroplasts: Regulation and Alternative Pathways of Electron Transfer. Biochemistry Moscow 88, 1438–1454 (2023). https://doi.org/10.1134/S0006297923100036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923100036