Abstract
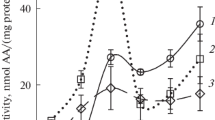
Protoplasts prepared from spinach leaves in May and June contained substantial amounts of ascorbate (1.33±0.28 μmol mg-1 chlorophyll), of which 30–40% was localised in the chloroplasts. During illumination, the ascorbate content was maintained at approximately the same concentration as in the dark in both protoplasts and chloroplasts, even in the absence of CO2 when pseudocyclic electron flow would be expected to be maximal. The addition of the Mehler reagent, methyl viologen, to isolated chloroplasts caused a rapid oxidation of stromal ascorbate in the light such that less than 95% of the ascorbate was oxidised after illumination for 1 min. Similarly the stromal ascorbate pool was rapidly oxidised upon the addition of H2O2. We conclude that when the intracellular ascorbate concentration is high, photosynthetically generated H2O2 can be reduced at rates comparable to its synthesis via the ascorbate-glutathione cycle. The addition of methyl viologen which catalyses rapid production of the superoxide anion, O -2 or the addition of excess H2O2, overwhelms the reductive cycle and the ascorbate system becomes partially or totally oxidised.
Similar content being viewed by others
References
Allen, J.F. (1977a) Oxygen-A physiological electron acceptor in photosynthesis. Curr. Adv. Plant Sci. 29, 459–469
Allen, J.F. (1977b) Effects of washing and osmotic shock on catalase activity of intact chloroplast preparations. FEBS Lett 84, 221–224
Allen, J.F., Whatley, F.R. (1978) Effects of inhibitors of catalase on photosynthesis and on catalase activity in unwashed preparations of intact chloroplasts. Plant Physiol. 61, 957–960
Anbar, M., Neta, P. (1967) A compilation of specific bimolecular rate constants for the reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals with inorganic and organic compounds in aqueous solution. Int. J. Appl. Radiat. Isot. 18, 493–523
Arnon, D.I. (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris L. Plant Physiol. 24, 1–15
Barker, J., Mapson, L.W. (1959) The ascorbic acid system in plant tissues. I. Influence of various methods of extraction in the estimation of dehydroascorbic acid. New Phytol. 58, 58–67
Black, C.C. (1966) Chloroplast reactions with dipyridyl salts. J. Exp. Bot. 120, 332–340
Bodannes, R.S., Chan, P.C. (1979) Ascorbic acid as a scavenger of singlet oxygen. FEBS Lett. 105, 195–196
Charles, S.A., Halliwell, B. (1980) Effect of hydrogen peroxide on spinach (Spinacia oleracea) chloroplast fructose bisphosphatase. Biochem. J. 189, 373–376
Dodge, A.D. (1982) Oxygen radicals and herbicide action. Biochem. Soc. Trans. 10, 73–75
Edwards, G.E., Robinson, S.P., Tyler, N.J.C., Walker, D.A. (1978) Photosynthesis by isolated protoplasts, protoplast extracts and chloroplasts of wheat. Influence of orthophosphate, pyrophosphate and adenylates. Plant Physiol. 62, 313–319
Foyer, C.H., Halliwell, B. (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133, 21–25
Franke, V.W., Heber, U. (1964) Über die quantitative Verteilung der Ascorbinsäure innerhalb der Pflanzenzelle. Z. Naturforsch. Teil B 19, 1146–1149
Fridovich, I., Hassan, H.M. (1979) Paraquat and the exacerbation of oxygen toxicity. Trends Biochem. Sci. 4, 113–115
Gerhardt, B. (1964) Untersuchungen über Beziehungen zwischen Ascorbinsäure und Photosynthese. Planta 61, 101–129
Giroud, A. (1938) L'acide ascorbique dans la cellule et les tissues. Borntrager, Berlin
Groden, D., Beck, E. (1979) H2O2 destruction by ascorbate-dependent systems from chloroplasts. Biochim. Biophys. Acta 546, 426–435
Halliwell, B. (1982) Ascorbic acid in the illuminated chloroplast. In: Ascorbic acid: chemistry, metabolism and uses. Advances in Chemistry, pp. 263–274, Seib, P.A., Tolbert, B.M., eds.
Halliwell, B., Foyer, C.H. (1978) Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta 139, 9–17
Halliwell, B., Foyer, C.H., Charles, S.A. (1981) The fate of hydrogen peroxide in illuminated chloroplasts. In: Photosynthesis II. Electron transport and photophosphorylation, pp. 279–284, Akoyunoglou, G., ed. Balaban International Science Services, Philadelphia
Heber, U., Takahama, U., Neimanis, S., Shimizu-Takahama, M. (1982) Transport as the basis of the kok effect. Levels of some photosynthetic intermediates and activation of light regulated enzymes during photosynthesis of chloroplasts and green leaf protoplasts. Biochim. Biophys. Acta 679, 287–299
Hewitt, E.J., Dickes, G.J. (1961) Spectrophotometric measurements on ascorbic acid and their use for the estimation of ascorbic acid and dehydroascorbic acid in plant tissue. Biochem. J. 78, 384–391
Jablonski, P.P. (1981) Reduction of hydrogen peroxide and selenite in illuminated pea chloroplasts. Ph.D. thesis, La Trobe University, Melbourne, Australia
Jablonski, P.P., Anderson, J.W. (1981) Light-dependent reduction of dehydroascorbate by ruptured pea chloroplasts. Plant Physiol. 67, 1239–1244
Jablonski, P.P., Anderson, J.W. (1982) Light-dependent reduction of hydrogen peroxide by ruptured pea chloroplasts. Plant Physiol. 69, 1407–1413
Kaiser, W. (1976) The effect of hydrogen peroxide on CO2 fixation of isolated intact chloroplasts. Biochim. Biophys. Acta 440, 476–482
Kelly, G.J., Latzko, E. (1980) Prospect of a specific enzymatic assay for ascorbic acid (Vitamin C). J. Agric. Food Chem. 28, 1320–1321
Law, M.Y., Halliwell, B. (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochem. J. (in press)
Lilley, R. McC., Walker, D.A. (1974) The reduction of 3-phosphoglycerate by reconstituted chloroplasts and by chloroplast extracts. Biochim. Biophys. Acta 368, 269–278
Lilley, R. McC., Fitzgerald, M.P., Rienits, K.G., Walker, D.A. (1975) Criteria of intactness and the photosynthetic activity of spinach chloroplast preparations. New Phytol. 75, 1–10
Mapson, L.W. (1958) Metabolism of ascorbic acid in plants: function. Annu. Rev. Plant Physiol. 9, 119–150
Marsho, T.V., Behrens, P.W., Radmer, R.J. (1979) Photosynthetic oxygen reduction in isolated intact chloroplasts and cells from spinach. Plant Physiol. 64, 656–659
Nakano, Y., Asada, K. (1980) Spinach chloroplasts scavenge hydrogen peroxide. Plant Cell Physiol. 21, 1295–1307
Nakano, Y., Asada, K. (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880
Nishikimi, M. (1975) Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem. Biophys. Res. Commun. 63, 463–468
Rabinowitch, E.I. (1945) Ascorbic acid in green plants. I. Ascorbic acid in the chloroplasts. In: Photosynthesis, vol. 1: Chemistry of photosynthesis, chemosynthesis and related processes in vitro and in vivo, pp. 269–271
Steiger, H.-H., Beck, E. (1981) Formation of hydrogen peroxide and oxygen dependence of photosynthetic CO2 assimilation by intact chloroplasts. Plant Cell Physiol. 22, 561–576
Takahama, U., Shimizu-Takahama, M., Heber, U. (1981) The redox state of the NADP system in illuminated chloroplasts. Biochim. Biophys. Acta 637, 530–539
Van Ginkel, G., Brown, J.S. (1978) Endogenous catalase and superoxide dismutase activities in photosynthetic membranes. FEBS Lett. 94, 284–286
Walker, D.A. (1980) Preparation of higher plant chloroplasts. Methods Enzymol. 69, 94–104
Whitehouse, D.G., Ludwig, L.J., Walker, D.A. (1971) Participation of the Mehler reaction and catalase in the oxygen exchange of chloroplast preparations. J. Exp. Bot. 22, 772–791
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Foyer, C., Rowell, J. & Walker, D. Measurement of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta 157, 239–244 (1983). https://doi.org/10.1007/BF00405188
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00405188