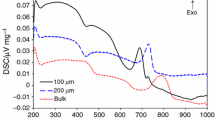
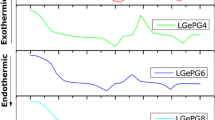
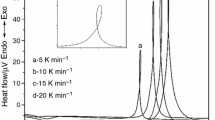
Abstract
This study examines the effect of MgO content on the crystallization behavior of lithium–aluminum–germanium–phosphate glasses using differential thermal analysis (DTA), X-ray diffraction (XRD), and scanning electron microscopy (SEM). The DTA results show that the crystallization temperature decreased with an increasing MgO content and increased with increasing heating rates. The crystalline phase is investigated with XRD to determine the presence of LiGe2(PO4)3 and LiMgPO4. The activation energy, as the kinetic parameter, is obtained using the Kissinger and Marotta methods, and the SEM results confirm the change in the Avrami index with the MgO content.







Similar content being viewed by others
References
Z. Ding, J. Li, J. Li, C. An, Review-interfaces: key issue to be solved for all solid-state lithium battery technologies. J. Electrochem. Soc. 167, 070541 (2020)
L. He, Q. Sun, C. Chen, J. Oh, J. Sun, M. Li, W. Tu, H. Zhou, K. Zeng, L. Lu, Failure mechanism and interface engineering for NASICON-structured all-solid-state lithium metal batteries. ACS Appl. Mater. Interfaces 11, 20895–20904 (2019)
H. Lim, L. Liu, H. Lee, J. Cha, D. Yoon, B. Ryu, The study on the interface characteristics of solid-stat electrolyte. J. Korean Ceram. Soc. 58, 373–377 (2021)
C. Jung, H. Shim, D. Eum, S. Hong, Challenges and recent progress in LiNixCoyMn1−x−yO2 (NCM) cathodes for lithium ion batteries. J. Korean Ceram. Soc. 58, 1–27 (2021)
H. Nakano, K. Dokko, J. Sugaya, T. Yasukawa, T. Matsue, K. Kanamura, All-solid-state micro lithium-ion batteries fabricated by using dry polymer electrolyte with micro-phase separation structure. Electrochem. Commun. 9, 2013–2017 (2007)
F. Croce, F. Serraino Fiory, L. Persi, B. Scrosati, A high-rate, long-life, lithium nanocomposite polymer electrolyte battery. Electrochem. Solid-State Lett. 4, 121–123 (2001)
K. Takada, M. Tansho, I. Yanase, T. Inada, A. Kajiyama, M. Kouguchi, S. Kondo, M. Watanabe, Lithium ion conduction in LiTi2(PO4)3. Solid State Ion. 139, 241–247 (2001)
T. Abe, M. Ohtsuka, F. Sagane, Y. Iriyama, Z. Ogumi, Lithium-ion transfer at the interface between lithium-ion conductive ceramic electrolyte and liquid electrolyte—a key to enhancing the rate capability of lithium-ion batteries. J. Electrochem. Soc. 151, 2151–2154 (2004)
A. Hayashi, T. Konishi, K. Tadanaga, M. Tatsumisago, Formation of electrode–electrolyte interface by lithium insertion to SnS–P2S5 negative electrode materials in all-solid-state cells. Solid State Ion. 177, 2737–2740 (2006)
X. Chen, P. Vereecken, Solid and solid-like composite electrolyte for lithium ion batteries: engineering the ion conductivity at interfaces. Adv. Mater. Interfaces 6, 1800899 (2019)
K. Heo, J. Im, J. Lee, S. Kim, J. Kim, J. Lim, High-rate blended cathode with mixed morphology for all solid-state Li-ion batteries. J. Electrochem. Sci. Technol. 11, 282–290 (2020)
T. Ozawa, Kinetics of non-isothermal crystallization. Polymer 12, 150–158 (1971)
J. Sestak, The applicability of DTA to the study of crystallization kinetics of glasses. Phys. Chem. Glasses. 15, 137–140 (1974)
H. Yinnon, D.R. Uhlmann, Applications of thermoanalytical techniques to the study of crystallization kinetics in glass-forming liquids, part I: Theory. J. Non-cryst. Solids 54, 253–275 (1983)
H.E. Kissinger, Reaction kinetics in differential thermal analysis. Anal. Chem. 29, 1702–1706 (1957)
Y. Zhang, Z. Luo, T. Liu, X. Hao, Z. Li, A. Lu, MgO-doping in the Li2O–ZnO–Al2O3–SiO2 glass–ceramics for better sealing with steel. J. Non-cryst. Solids 405, 170–175 (2014)
L. Lilensten, Q. Fu, B.R. Wheaton, A.J. Credle, R.L. Stewart, J.T. Kohli, Kinetic study on lithium-aluminosilicate (LAS) glass-ceramics containing MgO and ZnO. Ceram. Int. 40, 11657–11661 (2014)
H. Kim, The crystallization kinetics of CaO–MgO–Al2O3–sio2 glass system using thermal analysis. J. Korean Ceram. Soc. 29, 9–14 (1992)
J. Sestak, Use of phenomenological kinetics and the enthalpy versus temperature diagram (and its derivative-DTA) for a better understanding of transition processes in glasses. Thermochim. Acta 208(281), 175–190 (1996)
H.E. Kissinger, Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Stand. 57, 217–221 (1956)
A. Marotta, A. Buri, Kinetics of devitrification and differential thermal analysis. Thermochim. Acta 25, 155–160 (1978)
I. Jlassi, N. Sdiri, H. Elhouichet, Electrical conductivity and dielectric properties of MgO doped lithium phosphate glasses. J. Non-cryst. Solids. 466–467, 45–51 (2017)
J. Malek, The applicability of Johnson–Mehl–Avrami model in the thermal analysis of the crystallization kinetics of glasses. Thermochim. Acta. 267, 61–73 (1955)
A. Marotta, A. Buri, F. Branda, Nucleation in glass and differential thermal analysis. J. Mater. Sci. 16, 341–344 (1981)
A. Marotta, S. Saiello, F. Branda, A. Buri, Activation energy for the crystallization of glass from DDTA curves. J. Mater. Sci. 17, 105–108 (1982)
M. Kang, S. Kang, Influence of Al2O3 additions on the crystallization mechanism and properties of diopside/anorthite hybrid glass–ceramics for LED packaging materials. J. Cryst. Growth 326, 124–127 (2011)
B. Li, Y. Guo, J. Fang, Effect of MgO addition on crystallization, microstructure and properties of glass–ceramics prepared from solid wastes. J. Alloys Compd. 881, 159821 (2021)
H. Kun, W. Yanhang, Z. Chengkui, Z. Huifeng, L. Yonghua, C. Jiang, H. Bin, M. Juanrong, Influence of Al2O3 additions on crystallization mechanism and conductivity of Li2O–Ge2O–P2O5 glass–ceramics. Physica B Condens. Matter. 406, 2947–2950 (2011)
C.S. Ray, D.E. Day, W. Huang, K. Narayan, T.S. Cull, K.F. Kelton, Non-isothermal calorimetric studies of the crystallization of lithium disilicate glass. J. Non-cryst. Solids. 204, 1–12 (1996)
M. Fathollahi, S. Pourmortazavi, S. Hossenini, Particle size effects on thermal decomposition of energetic material. J. Energ. Mater. 26, 52–69 (2008)
A. Arora, E.R. Shaaban, K. Singh, O.P. Pandey, Non-isothermal crystallization kinetics of ZnO–BaO–B2O3–SiO2 glass. J. Non-cryst. Solids. 354, 3944–3951 (2008)
M. Ghasemzadeh, A. Nemati, A. Nozad Golikand, Z. Hamnabard, S. Baghshahi, Utilization of DTA in the determination of a crystallization mechanism in transparent glass–ceramics with a nanocrystalline structure. Inorg. Nano-Met. Chem. 41, 561–570 (2011)
C.R. Chang, J.H. Jean, Crystallization kinetics and mechanism of low-dielectric, low-temperature, cofirable CaO–B2O3–SiO2 glass-ceramics. J. Am. Ceram. Soc. 82, 1725–1732 (1999)
M. Avrami, Kinetics of phase change: I. General theory. J. Chem. Phys. 7, 1103–1112 (1939)
M. Avrami, Kinetics of phase change: II. Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 8, 212–224 (1940)
M. Avrami, Kinetics of phase change: III. Granulation, phase change and microstructure. J. Chem. Phys. 9, 177–184 (1941)
X.J. Xu, C.S. Ray, D.E. Day, Nucleation and crystallization of Na2O · 2CaO · 3SiO2 glass by differential thermal analysis. J. Am. Ceram. Soc. 74, 909–914 (1991)
A. Das, M. Goswami, M. Krishnan, Crystallization kinetics of Li2O–Al2O3–GeO2–P2O5 glass–ceramics system. J. Therm. Anal. Calorim. 131, 2421–2431 (2018)
C.S. Ray, D.E. Day, Ceramic Transactions Nucleation and Crystallization in Liquids and Glasses (The American Ceramic Society, Ohio, 1993), pp. 207–223
Z. Lu, J. Lu, X. Li, G. Shao, Effect of MgO addition on sinterability, crystallization kinetics, and flexural strength of glass-ceramics from waste materials. Ceram. Int. 42, 3452–3459 (2016)
J. Li, Y. Guo, G. Li, J. Chen, C. Li, Y. Zou, Investigation into the role of MgO in the synthesis of MAPO-11 large single crystals. Microporous Mesoporous Mater. 79, 79–84 (2005)
S.M. Salman, S.N. Salama, H. Darwish, E.A. Mahdy, The role of MgO on the structural properties of CaO–Na2O(MgO)–P2O5–CaF2–SiO2 derived glass ceramics. Ceram. Int. 36, 55–61 (2010)
Acknowledgements
This study was financially supported by the 2020 Post Doc. Development Program of Pusan National University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cha, JM., Liu, L., Lee, HJ. et al. Crystallization kinetics of lithium–aluminum–germanium–phosphate glass doped with MgO using a non-isothermal method. J. Korean Ceram. Soc. 58, 614–622 (2021). https://doi.org/10.1007/s43207-021-00137-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-021-00137-1