Abstract
Investigations on the microstructural and electrical properties of a glassceramic system: (100 − x) [0.4Li2O − 0.1GeO2 − 0.6P2O5] + x [20 h ball milled Ga2O3] (0 ≤ x ≤ 10 mol%, in the regular steps of 2 mol%) via high-energy ball milling technique are reported. XRD spectra of LGePG glassceramic samples were identified major crystalline phases such as Li3Ge2(PO4)3 (NASICON-type phase), Ga2Li3(PO3)7 and GeO2 from the major diffraction peaks. Bulk conductivity of all these samples was measured for the powder-compressed pellets by ac impedance method. The correlation of log(σ) and M″ peaks suggests the presence of single conduction mechanism in the LGePG glassceramic samples. The results are discussed in the light of degree of crystallization of lithium phosphate glass network.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, solid-state glassceramic electrolytes when precipitated with superionic nanocrystals, they got attracted because of their remarkable electrical conductivity (Al-Hartomy et al. 2013; Fanelli et al. 2011; El-Desoky 2010). Among the oxide and sulfide-based glassceramic electrolytes, the ionic conductivity of sulfide-based compounds is superior over oxide based counter parts. However, sulfides are not chemically stable over longer durations than oxides (Kim et al. 2008, Kim and Jeong 2011). Oxide-based glassceramic electrolytes are highly preferred because they posses excellent chemical and mechanical stability even under heavy loads, nevertheless, their ionic conductivity barely meets the required power densities of the cell (Kim et al. 2008, Kim and Jeong 2011; Hayashi et al. 2012). On the other hand, NASICON-based glassceramic type structure was given top priority as it facilitates the fast diffusion pathways for monovalent ions which is the necessary and sufficient condition for boosting the conductivity at least by two orders (He et al. 2014).
Though, several routes were taking place to enhance ionic conductivity amorphous electrolyte materials, precipitation of nanocrystals in the glass and glassceramic materials via high-energy ball milling was regarded as highly efficient choice, where nanoscaled powders of electrodes directly mixed with electrolytes, leads to reduce the interfacial resistance and also surface modification under room temperature and normal pressure (Kang et al. 2003; Xu and Gao 2004; Lam et al. 2004; Dell et al. 2007; Reddy et al. 2012). Furthermore, it is also to be noted down that the uniform distribution of fine grains of nanocrystalline phases in the glass and glassceramic structure can significantly produce fast diffusion path ways and hence, desirable ionic conductivity is expected to achieve (Dell et al. 2007; Reddy et al. 2012). Ga2O3 is a good chemically and thermally stable material whose quantum size effects will influence largely on Li–Ge–P network for its high Li ion conductivity, thermal stability, and physical properties (Prasad et al. 2005). On the other hand, the role of GeO2 in this glassceramic matrix significantly improves the phase immiscibility during its transition from glass to the glassceramic matrix which is a fundamental property to control the degree of crystallization. In view of this, correlation between the mechanism of crystallization and its electrical transport is worthwhile to discuss for the use in the high-energy density applications (Prasad et al. 2005).
We have already reported in our investigation that the estimated mean sizes for the 20 h ball milled Ga2O3 nanocrystallites are obtained by using the Scherrer’s formula for half width of the diffraction peaks of the all the in the lithium titatanate phosphate glass ceramic samples vary in between 12 and 17 nm along with little larger clusters ~20–50 nm in size for the 20 h ball mill which was chosen for dispersion in L–Ge–P glassceramic matrix. However, beyond 20 h the powder gets strained (Reddy et al. 2012).
Experimental
The glassceramic samples with general formulae: (100 − x) [0.4Li2O − 0.1GeO2 − 0.6P2O5] + x [20 h ball milled Ga2O3]; (0 ≤ x ≤ 10 mol%, in the steps of 2 mol%) have been synthesized by high-energy ball milling technique, labeled as LGePG x (where x is the mol% of 40 h ball milled Ga2O3) from the mixtures of analytical reagent-grade chemicals of Li2CO3, P2O5, GeO2 and Ga2O3. Glassceramic samples are prepared by heating the mechanically milled glasses over their crystallization temperatures. The synthesis procedure to conceive final powders and their green pellets is as published from our laboratory (Reddy et al. 2012). Green pellets thus obtained were sintered over their crystalline temperatures at 2 °C/min and then cooled down to room temperature in the furnace without any plateau stage. The DTA traces were recorded in the temperature range of 300–1300 K using analytical grade Al2O3 as a reference material by using DT-30 Schimadzu thermal analyzer (error ±2 K) in flowing nitrogen by heating at 20 K/min. The degree of crystallization of phases was studied by XRD method using PANanalytical Diffractometer B.V fitted with Cu target (both Kα 1 + α 2 wavelengths) and Ni filter at 40 kV and 30 mA (2θ range). The electrical conductivity studies were carried out using a computer-controlled Solatron 1260 Impedance/Gain phase analyzer with pellet specimens of 10 mm diameter and about 3 mm thick. The silver coating was applied on both sides of pellet. Measurements were made conducted in the frequency range of 10–106 Hz at room temperature and as well as temperatures from 303 to 423 K with a 10 K temperature step in dry Ar atmosphere.
Results and discussion
DTA patterns obtained for all the LGePG glassceramic samples are shown in the Fig. 1. It is to be noted that crystallization phenomenon can be treated as an exothermic process in glasses and glassceramic materials in view of the fact that the free energy of crystalline phase is lower than that of glassy phase. The exothermic peak related to the crystallization temperatures T c (tangent at the inflexion point) for the possible crystalline phases varies from 877 to 841 K. Where as endothermic related to glass transition T g (intercept of the base line) changes from 802 to 751 K from the sample LGePG0 to LGePG6, however, from LGePG8 to LGePG10, the trend is reversed. The glass transition temperature (T g) decreases from LGePG0 to LGePG6 which shows their increasing trend of de-polymerization. However, increasing trend in the lattice strain is indicated by the increase in the glass transition temperature (T g) from the LGePG6 (751 K) to LGePG10 (791 K). Nevertheless, the glass stability parameter (K gl) is a good estimate of the glass forming tendency; higher is K gl value, more stable is the glass and less critical is the quenching rate. It can be calculated from the DTA parameters using the relation (K gl = (T c − T g)/(T m − T c)) and it should be noted down that for good glass forming systems, the value of K gl should be greater than or equal to unity (Hruby 1972). The details of DTA parameters for all the LGePG samples are listed in Table 1. The decreasing trend in the value of K gl from LGePG0 (0.620) to LGePG6 (0.579), denotes the decrease in the degree of de-polymerization, however, from LGePG8 to LGePG10, K gl is observed to increase. Though the K gl of LGePG6 is low compared to other samples of LGePG series, it has become our choice of interest because of two reasons: (a) low glass transition temperature (751 K) that would exhibit the good electrical properties, (b) optimum degree of crystallization by the precipitation of nanocrystallne phases which would yield correlation between the mean bond strength and cross-link density of the glassceramic networks. Hence, the transformation of amorphous phase (dis-order) into crystalline (order) expected to influence largely on the conduction features as a function of DTA parameters.
Our earlier investigation reported that Ga2O3 crystallites after 20 h of milling were precipitated with crystalline phases with optimum crystallization in glassceramic matrix (Reddy et al. 2012). XRD powder diffraction patterns were obtained on all the LGePG glassceramic samples at room temperature is to determine the phase compositions (Fig. 2). Broad crystalline peaks together with other broad and weak clusters of peaks centered at various 2θs in the patterns of all the samples attributed to the secondary phases with variable intensity. The nanocrystalline phases such as LiGe2(PO4)3 (NASICON type) and, Ga2Li3(PO3)7 were identified from major diffraction peaks of LGePG samples (Reddy et al. 2015) and these phases were justified by the first and second exothermic peaks in DTA traces (Fig. 1). Nevertheless, there are some weak peaks corresponding to the GeO2 phase and some very weak peaks which are yet to be identified. The NASICON-type phase of LiGe2(PO4)3 is observed predominantly as depicted in Fig. 2, and its intensity increases with Ga2O3 content. Distances between XRD peaks of the samples and the horizontal axis were measured at various 2θs (JCPDS File, Card no: 80-1922). The volume fraction of crystallization and relative to other for all the LGePG glassceramic samples was obtained by the Ohlberg and Strickler equation (Ohlbeg and Stricklet 1962). The average percentage of crystallization at various 2θs in all the LGePG glassceramic samples is shown in Table 2. The aluminum is absent in this type of phase and there is an every possibility for the partial substitution of ions having similar ionic radii such as Ge4+ by Al3+ during the transformation of glass into glassceramics which induces more Li+ ions into the crystal structure, leading to boost the ionic conductivity in the present LGePG glass system. However, the massive crystallization in these glassceramic samples resulting to reduce the conducting features. One more conducting phase Ga2Li3(PO3)7 was also recorded in all the LGePG glassceramic samples which boosts up the electrical conductivity in addition with NASICON-type phase which appears as second exothermic peak in DTA trace (Fig. 1). On the other hand, the GeO2 phase is identified as the most insulating phase, whose intensity is almost constant for all the samples of LGePG glassceramic system (Kim et al. 2006). As shown in Table 2, the secondary GeO2 phase has appeared with small volume fraction (2.58 %) out of the average crystallization percentage of NASICON-type phase in LGePG6 sample (Table 3) which does not affect much on the overall mechanism of crystallization of glass. The analysis reflects that the predominant NASICON-type phase (LiGe2(PO4)3) which has been characterized as rhombohedral crystal system with a unit cell of dimensions a = 8.2643 Å, b = 8.2645 Å, c = 2.5681 Å and β = 98.99°, which is in agreement with the reported data (JCPDS card) and space group R3c. However, correlation between the quantitative volume fraction of crystalline phases (Table 3) and their electrical features of the LGePG glassceramic samples will be discussed in the next section.
SEM images of some samples are crucial to analyse crystallization behavior by considering the phase and microstructural details (Fig. 3). It can be seen from the Fig. 3, that crystalline phase appears with white region where as residual glass phase appears with black region. Figure 3a, c shows the presence of agglomerated grain like crystallites (170–300 nm) of major crystalline phases [LiGe2(PO4)3 (NASICON type), and Ga2Li3(PO3)7] precipitated along the grain boundaries. SEM image of LGePG6 glassceramic sample (Fig. 3b) revealed the presence of interconnected fine and uniform grain crystallites (110–170 nm) of all the as mentioned major crystalline phases which are uniformly dispersed in the glass matrix and separated by well-developed grain boundaries throughout the micrograph. XRD pattern (Fig. 2) justified these grain-like crystallites belong to the major crystalline phases [LiGe2(PO4)3 (NASICON type), and Ga2Li3(PO3)7]. Nevertheless, it can be seen from Fig. 3b that the crystal growth of crystallites is optimum and distributed by connective tissues of the LGePG6 glassceramic matrix among all the samples under investigation. On the other hand, crystal growth of the crystallites is not perfect and uniform for rest of all other samples as synthesized. To get better conducting features via the presence of the ionically conductive, interfacial region between the glassy matrix and devitrified crystalline grain, further investigations require on the heat treatment schedule around the glass transition temperature (T c) of these LGePG glassceramic samples.
The electrical properties of as synthesized glass and glassceramic samples were monitored using impedance spectroscopy (Kupracz et al. 2015). This technique is relatively powerful to investigate the dynamics of mobile charges in the bulk or interfacial regions of any kind of materials which operates an alternating current with variable frequency. The impedance data obtained were fitted to nonlinear least-square method. Here, the electrical properties can be associated to a parallel resistance and capacitance circuit corresponding to equivalent to the individual component of the materials (i.e., bulk and grain boundary) represents a semicircle and therefore bulk response of the sample is detected. Hence, the bulk conductivity may be evaluated for all the LGePG glassceramic samples from the impedance spectrum using the relation
where R is the bulk resistance which was determined from the intersection of the semicircle with the real axis of the impedance Z′; t the thickness, and A the surface area of the sample and is achieved to be highest for LGePG6 sample (σ = 8.31 × 10−04 S/cm) at 303 K (Fig. 4). Compositional dependence of glass stability parameter (K gl) and bulk conductivity (σ b) for all the LGePG glassceramic samples are depicted in Fig. 4. The lowest value of glass stability parameter (K gl) for the highest conducting LGePG6 sample indicates that optimum degree of its crystallization achieved by the precipitation of nanocrystalline phases boosting the conductivity features as justified by XRD patterns and SEM images (Milankovic et al. 1997; Garbarczyk et al. 2006).
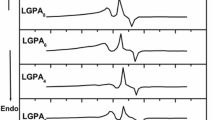
The Nyquist plots between (Z″ vs Z′) for all the LGePG glassceramic samples measured at 303 K and for highest conducting sample LGePG6 at different temperatures are shown in Figs. 5 and 6. The impedance spectrum was found to exhibit single semicircular arc which shows that the electrical properties of present samples arises due to bulk effects. Bulk conductivity of all the glassceramic samples at different temperatures (303–423 K) is obtained by using the resistance (R) obtained from the analyzed impedance data and pellet dimensions. It can be seen that increase in temperature caused the impedance semicircles to be shifted to lower and lower Z′ values which indicates the decrease in the resistive property of the material (Fig. 6).
The conductivity data are fitted to the Arrhenius equation
σ 0 is the pre exponential factor and E aσ , k and T are the activation energy for the conduction, Boltzman’s constant (1.3806 × 10−22 J/K) and absolute temperature, respectively. The activation energy for conduction (E aσ ) was calculated from the slope of the straight lines obtained when the data is plotted against 1000/T of all the glassceramic samples. The slope is calculated as follows. (−E aσ /kT) is the slope and Eq. (2) can be written as
and the value of slope can be calculated from the linear graph of ln(σ) vs \(\frac{1}{T}\) as follows
The activation energy for conduction (E aσ ) is now calculated as E aσ = −k × slope. The slope being negative, a positive value of E aσ is obtained.
The compositional dependence of activation energy for conduction (E aσ ) for all the LGePG glassceramic samples at 303 K is shown in the Fig. 7. It is observed that the activation energy is found to be the lowest for the highest conducting sample LGePG6 (Fig. 7) and it does not show the nonlinear behavior of conductivity which reveals that the variation of mobile Li+ ion conductivity is directly related to the percentage of crystallization (Panmand et al. 2010; Raghavan and Rao 1996; Xiaoxiong et al. 2006). The same has been justified by the intensity and broadening of XRD peaks of LGePG6 among all the samples under investigation with optimum crystallization (58.9 %). Hence, the increased mobility of Li+ ions in addition to the percentage of crystallization plays a vital role in boosting the conductivity of the LGePG series of samples (Heitjansa et al. 2008; Garbarczyk et al. 2006).
The frequency-dependent electrical conductivity fulfills the power-law equation
where characteristic parameter ‘s’ lies in between zero and one and decreases slightly with increasing temperature. In general, the low-frequency part of the conductivity is frequency independent and the frequency-independent conductivity (σ o ) is obtained by extrapolation of the conductivity approaches to the frequency as zero. Conductivity exhibits the dispersion at the high-frequency region and increases in a power-law fashion (Nobre and Lafendri 2001). AC conductivity plots as a function of frequency for all the LGePG samples at 303 K are shown in Fig. 8. It is to be noted down that change in the slope of ac conductivity with frequency concludes domination of grain resistance over grain boundary resistance for a particular temperature (Sambasiva Rao et al. 2008). The small deviation from σ dc in the frequency-independent part of the conductivity reflects the influence of electrode polarization in the glassceramic samples. Critical frequency is frequency (ω p = f o ), at which the conductivity deviates from the frequency-independent part. The shift in the critical frequency towards higher frequency side on increasing temperature is a little more obvious for the ac conductivity of LGePG6 at different temperatures (Fig. 9). Nevertheless, the relation between frequency-independent conductivity σ 0 and the critical frequency (f o ) can be represented by the following equation:
where K = (Ne 2 a 2/kT)νc(1 − c), is the empirical constant that depends on the concentration of mobile ions, temperature and the conduction mechanism (Jain et al. 1993). In the equation for K, ν is the geometrical factor which includes the correlated factor, c is the concentration of mobile ions on N equivalent lattices per unit volume, a is the hopping distance, e is the electronic charge, T is absolute temperature and k is Boltzman’s constant. The relaxation effects begin to appear at the critical frequency (f o ), which moves towards the higher frequency with an increase in the temperature of the measurement. Further, it is observed that the critical frequency (f o ) is thermally activated with the same activation energy as that of the conductivity σ 0 T (Verhoef and den Hartog 1994).
Compositional dependence of characteristic parameter (s) is known to be material dependent and its value is observed to be the lowest for highest conducting sample LGePG6 (Fig. 7). A summary of the electrical conductivity, activation energy and characteristic parameters is given in Table 4. The frequency-independent part and dependent parts of the conductivity may be explained by long-range transport of Li+ ions and diffusion controlled relaxation model (Elliot and Owens 1994).
To explore the further information about the dependence of the ion transport on the composition, temperature, structure, and also on the concentration of the charge carriers, the ac conductivity data obtained at various temperatures for LGePG6 at different temperatures have been scaled and are presented in the inset of Fig. 9 (Karmakar et al. 2009). Perfect overlapping of normalized imaginary part of AC conductivity plots of LGePG samples suggested the conduction transport mechanism to be temperature independent. The contribution of highly conductive phases such as NASICON-type phase of LiGe2(PO4)3 in addition with another conducting phase Ga2Li3(PO3)7 is boosting the ac conductivity from LGePG0 to LGePG6 (Kun et al. 2011). On the other hand, the highest conductivity is achieved to be (σ = 8.31 × 10−04 S/cm) for LGePG6 sample which is expected due to the optimum percentage of crystallization 58.9 % (Table 2) and the presence of uniform distribution of conducting crystalline phases. However, the massive crystallization results to decrease the conductivity from LGePG8 to LGePG10 (Garbarczyk et al. 2006). In this process, we have monitored variation of imaginary permittivity (ε″) with frequency, where the imaginary part ε″ is exceeded by the dc conductivity and will not be discussed further and hence, the modulus formalism was used for the further analysis.
Electrical modulus formulation is a convenient tool to explore electrical transport phenomenon of the material. Also, it pacifies the noise of extrinsic relaxation often used in the analysis of electrode polarization and bulk phenomena such as average conductivity relaxation times τ σ of ionically conducting glasses (Mc Crum et al. 1967; Moynihan 1994; Moynihan et al. 1973).
It is speculated that the simultaneous application of both impedance (Z″ vs Z′) and modulus (M″ vs M′) spectroscopy would deal to rationalize the dielectric properties (Sinclair and West 1989, 1997). In view of technological importance, we have analysed both electrical conductivity and modulus formalisms simultaneously, in this investigation. Figures 10 and 11 show the frequency dependence of imaginary part of electric modulus M″ for all the LGePG samples at 303 K and for highest conducting sample LGePG6 at different temperatures (303–423 K). The Μ″ value is observed to be constant (Μ″max) at a relaxation frequency (f max) and the peak Μ″max symmetrically shifts towards higher frequencies with increasing temperatures (Fig. 11). This behavior suggests that the dielectric relaxation is not the usual thermally activated type in which hopping mechanism of charge carriers dominates intrinsically. From Fig. 10, the relaxation frequency (f max) corresponding to the maximum in Μ″ curves and the relaxation time τ, calculated using the relation τ = 1/2π f max at different temperatures can be obtained and presented in Table 4. Nevertheless, the activation energy for the conductivity relaxation E aτ for all the LGePG samples was calculated from the relaxation time plots and the values of Ε aτ and Ε aσ are in good agreement with each other (Table 4) which suggest that the ionic-hopping mechanism responsible for the relaxation is the same as that for the conductivity (Chowdari et al. 1990). In principle, full width half maximum (β) can be calculated by the width at the half maxima for any bell shaped curve and the same method was adopted for all the frequency-dependent electrical modulus curves (Fig. 10). The full width half maximum (β) and characteristic relaxation time (τ) of Μ″ peaks are given in Table 4. The value of β is lowest for the highest conducting sample LGePG6, which justifies its optimum percentage of crystallization as supported in the XRD analysis leading to modify the conductivity in the neighborhood of conduction path. The overlap of critical frequency (f o) of log(σ) peaks and relaxation frequency of and Μ″ peaks for the highest conducting sample LGePG6 at different temperatures (323, 363 and 423 K) have been demonstrated to be a result from the same mechanism and this result is further confirmed that the two processes are closely correlated with each other (Fig. 12). As the temperature increases, the same corresponding peak shifts to a higher frequency but they present the same characteristic relaxation time at specific temperatures. Nevertheless, the peak in log(σ) is slightly shifted to a higher frequency with respect to the Μ″ peak position in all LGePG samples which suggests the presence of possibly more than one mechanism but this contribution does not influence the overall conductivity response that much as it was determined that the activation energies calculated from the conductivity curves and those of the relaxation were essentially the same. This is supported by master curves in the normalized plots of electric modulus spectra (inset of Fig. 11), which suggests that the dynamical relaxation processes in the LGePG samples is temperature independent. The β values show that the conductivity relaxation is to be highly nonexponential with increasing temperature (Table 4).
Conclusions
The lowest value of K gl for the glassceramic sample LGePG6 (0.517), denotes the decrease in the degree of de-polymerization which possesses fast diffusion pathways, leading to achieve good compatibility between electrodes. XRD patterns pointed out that the LGePG6 sample exhibits the optimum crystallization of nanocrystalline phases such as LiGe2(PO4)3 (NASICON type), Ga2Li3(PO3)7 from major diffraction peaks. SEM pictures revealed that LGePG6 glassceramic sample is observed to be interconnected fine and uniform crystallites of major crystalline phases (LiGe2(PO4)3 (NASICON type), Ga2Li3(PO3)7) which are uniformly dispersed in the glass matrix and separated by well-developed grain boundaries which is justified by XRD pattern. The bulk conductivity is achieved to be the highest for LGePG6 sample (8.31 × 10−04 S/cm). The M″ spectra shows non-Debye type relaxation and shift to higher frequency side for the sample LGePG6 with the highest conductivity due to the increase of mobile charge carriers and the same would be applicable for all the other samples under investigation. The modulus scaling spectra show that the dynamical relaxation processes is temperature independent for a particular composition. The β exponent value indicates that the conductivity relaxation is highly nonexponential (Table 4).
References
Al-Hartomy OA, Al-Ghamdi AA, El-Tantawy F, El-Desoky MM (2013) Effect of nanocrystallization on the structural and electrical conductivity enhancement of vanadium-based glasses. J Mater Sci 48:3067–3074
Chowdari BVR, Krishnan RG, Ghosh SH, Tan KL (1990) Thermal, electrical and structural characterization of Li2O·P2O5·MoO3 glasses. Solid State Ionics 40–41(2):684–688
Dell LAO, Savin SLO, Chadwick AV, Smith ME (2007) Multi nuclear MAS NMR investigation of sol–gel and ball milled nanocrystalline Ga2O3. Appl Mag Reson 32:527–546
El-Desoky MM (2010) Gaint electrical conductivity enhancement in BaO-V2O5-Bi2O3 glass by nanocrystallization. Mater Chem Phys 119:389–394
Elliot SR, Owens AP (1994) Frequency-dependent conductivity in ionically and electronically conducting amorphous solids. Solid State Ionics 70:27
Fanelli E, Pernice P, Xiao M, Sigaev VN (2011) Crystallization behaviour and nanostructuring in alkali niobiosilicate glasses. J Mater Sci Technol 27(2):189–192
Garbarczyk JE, Jozwiak P, Wasiucionek M, Nowinski JL (2006) Effect of nanocrystallization on the electronic conductivity of vanadate–phosphate glasses. Solid State Ionics 177:2585–2588
Hayashi A, Noi K, Sakuda A, Tatsumisago M (2012) Superionic glassceramic electrolytes for room-temperature rechargeable sodium batteries. Nat Commun 3:856–860
He K, Zu C, Wang Y, Han B, Yin X, Zhao H (2014) Stability of lithium ion conductor NASICON structure glass ceramic in acid and alkaline aqueous solution. Solid State Ionics 254:78–81
Heitjansa P, Tobschallb E, Wilkening M (2008) Ion transport and diffusion in nanocrystalline and glassy ceramics Eur. Phys J Spec Topics 161:97
Hruby A (1972) Evalution of glass-forming tendency by means of DTA. Czech J Phys B 22(11):1187–1193
Jain H, Kanert O (1993) In: Spaeth JM (eds) Proceedings of the XII international conference on defects in insulting materials, vol 1. World Scientific Co, p 274
Kang D, Han M, Lee SG (2003) Dielectric and pyroelectric properties of barium strontium calcium titanate ceramics. J Eur Cerm Soc 23(3):515–518
Karmakar A, Majumdar S, Giri S (2009) Polaron relaxation and hopping conductivity in LaMn1−xFexO3. Phys Rev B 79:094406/1–094406/7
Kim H-S, Jeong C-S (2011) Electrochemical properties of binary electrolytes for lithium-sulfur batteries. Bull Korean Chem Soc 32:3682–3686
Kim Y, Saienga J, Martin SW (2006) Anomalous ionic conductivity increase in Li2S–GeS2–GeO2 glasses. J Phys Chem B 110(33):16318–16325
Kim Y, Hwang H, Lawler K, Martin SW, Cho J (2008) Electrochemical behavior of Ge and GeX2 (X = O, S) glasses: improved reversibility of the reaction of Li with Ge in a sulfide medium. Electrochim Acta 53:5058–5064
Kun HE, Yan-Hang W, Cheng-Kui Z, Yong-Hua L, Hui Feng Z, Bin H, Jiang C (2011) Microstrucutre and Ionic conductivity of Li2O–Al2O3–GeO2–P2O5 glassceramics. Chin J Inorg Chem 27(12):2484–2488
Kupracz P, Karczewski J, Prześniak-Welenc M, Szreder NA, Winiarski MJ, Klimczuk T, Barczy’nski RJ (2015) Micrsostructure and electrical properties of manganese borosilicate glasses. Non-Cryst Solids 423–424:68–75
Lam HM, Hong MH, Yuan S, Chong C (2004) Growth of β-Ga2O3 nanoparticles by pulsed laser ablation technique. Appl Phys A 79(8):2099–2102
LiGe2 (PO4)3—JCPDS powder diffraction file, card no. 80-1922. Joint Committee on Powder diffraction Standards (JCPDS), Swarthmore
Mc Crum NG, Read BE, Williams G (1967) Anelastic and dielectric effects in polymeric solids. Wiley, New York
Milankovic AM, Furic K, Day DE (1997) Raman studies of PbO–Bi2O3–Ga2O3 glasses and crystallized compositions. Phys Chem Glasses 38:148–150
Moynihan CT (1994) Analysis of electrical relaxation in glasses and melts with large concentrations of mobile ions. J Non-Cryst Solids 172–174:1395–1407
Moynihan CT, Boesch LP, Laberge NL (1973) Decay function for the electric field relaxation in vitreous ionic conductors. Phys Chem Glasses 14:122–125
Nobre MAL, Lafendri S (2001) Phase transition in sodium lithium niobate polycrystal: an overview based on impedance spectroscopy. J Phys Chem Solids 21:999
Ohlbeg SM, Stricklet DW (1962) Effects of composition changes on the crystallization behavior and properties of SiO2–Al2O3–CaO–MgO (Fe2O3–Na2O–K2O). J Am Ceram Soc 45:170–172
Panmand RP, Kawade UV, Kulkarni MV, Apte SK, Kale BB, Gosavi SW (2010) Synthesis and characterization of Bi2S3 nanosrystals in glass matrix. Mater Sci Eng B 168:161
Prasad SVGVA, Bhaskaran GS, Veeraiah N (2005) Spectroscopic, magnetic and dielectric investigations of BaO–Ga2O3–P2O5 glasses doped by Cu ions. Phys Sta Soli A 202:2812–2828
Raghavan MS, Rao KJSL (1996) Electrical transport studies in alkali borovanadate glasses. J Phys Chem 100:4243
Reddy CKK, Balaji Rao R, Koti Reddy CV (2012) Influence of nanocrystalline phases on the electrical properties of lithium titanate phosphate glassceramics mixed with Ga2O3 nanocrystals. J Phase Transit 85:218
Reddy CKK, Balaji Rao R, Gopal Reddy C (2015) The role of crystallization on microstructural and electrical studies of lithium germanium phosphate glass-ceramic electrolytes. Ionics 21:967–979
Sambasiva Rao K, Murali Krishna P, Swarna Latha T, Madhava Prasad D (2008) Impedance spectroscopy analysis and piezoelectric properties of Pb2KNb5O5 ceramics. Mater Sci Eng B 131(1):127
Sinclair DC, West AR (1989) Impedance and modulus spectroscopy of BaTiO3 showing positive temperature coefficient of resistance. J Appl Phys 66:3850–3856
Sinclair DC, West AR (1997) Characterization of electrical materials, especially ferroelectrics, by impedance spectroscopy. J Electroceram 1(1):65–71
Verhoef AH, den Hartog HW (1994) High frequency dielectric properties of alkali and alkali-halide borate glasses. Solid State Ionics 68:305
Xiaoxiong XU, Zhaoyin W, Xuelin Y, Gu Z (2006) Ionic conductivity and microstructural evaluton of Li2O–TiO2–P2O5–SiO2 glass ceramics. Solid State Ionics 177:2611
Xu H, Gao L (2004) Hydrothermal synthesis of high purity BaTiO3 powders: control of powder phase and size, sintering density and dielectric properties. Mater Lett 58:1582–1586
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Reddy, C.K.K., Suman, G., Balaji Rao, R. et al. Lithium–germanium–phosphate glassceramic electrolytes: correlation between the nanocrystallization and electrical studies. Appl Nanosci 6, 1043–1052 (2016). https://doi.org/10.1007/s13204-016-0519-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-016-0519-x