Abstract
In nature, bees with damaged tongues are adapted to have a feat in collecting nectariferous sources in a large spectrum of concentrations (19%–69%) or viscosities (10–3 Pa·s to 10–1 Pa·s); however, effects of nectar property on compensated dipping behavior remain elusive. Combining the bee tongue anatomy, high-speed videography, and mathematical models, we investigate responses of honey bees with damaged tongues to fluidic sources in various properties. We find that, bees with 80% damaged tongues are deprived of feeding capability and remarkably, the dipping frequency increases from 4.24 Hz to 5.08 Hz while ingesting 25% sugar water when the tongue loses 0–30% in length, while declines from 5.08 to 3.86 Hz in case of 30% damaged tongue when sucrose concentration increases from 25% to 45%. We employ the energetic compensation rate and energetic utilization rate to evaluate effectiveness of the compensation from the perspective of energetic regulation. The mathematical model indicates that the energetic compensation rate turns higher in bees with less damaged tongues for ingesting dilute sugar water, demonstrating its capability of functional compensation for combined factors. Also, the tongue-damaged bees achieve the highest energetic utilization rate when ingesting ~ 30% sugar water. Beyond biology, the findings may shed lights on biomimetic materials and technologies that aim to compensate for geometrical degradations without regeneration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Based on physiological and environmental constraints, animals can improvise drinking strategies using a wide spectrum of mouthparts [1,2,3,4]. Recently, fluid transport in animals is raising great curiosity in both biologists and physicists. For instance, the domestic cat uses its tongue tip to generate fluid inertia and pull liquid into the mouth by defeating gravity [1]. Likewise, the dog curls its tongue ventrally in a ladle shape to impale the liquid surface and then retracts and pulls upward in the mouth with fluid adhering to the dorsal side of the tongue, exploiting the fluid dynamics to generate a water column [4]. Some insects have specialized mouthparts to transport fluid with a variety of physical properties. For instance, the butterflies and mosquitoes ingest fluid by suction through slender proboscis [3, 5]. As one of the most important flower-visiting insects, the honey bee has a bushy hairs coated compliant and segmented tongue to imbibe nectar [6, 7]. The back-and-forth movement of the honey bee’s hairy tongue helps loading nectar from flowers [8, 9]. In general, a bee visits about 3000 flowers in a single day and collects over a period of 10–20 days [10]. Notably, a honey bee stays at a corolla for about 11 s and dips with a frequency of about 5 Hz [11]. In aggregate, a honey bee makes about 2.5 million dipping cycles in its entire life span [10, 12]. Given the high-intensive work of collecting nectar, tongue damage in honey bees is inevitable and has been verified by field tests [13].
In nature, damage to animal appendages occurs commonly, causing functional degradation even catastrophic structural failure. Fascinatingly, some animals have evolved specialized technologies to compensate for such deterioration [13,14,15,16,17]. Moon jellyfish responds to injuries by reorganizing existing parts to rebuild essential body symmetry, without regenerating the lost part [17]. Upon losing a siphon or an arm to predators, clam, and brittlestar alter their feeding behavior and efficiency to compensate and regenerate the lost body part [14,15,16]. Similarly, insects have evolved with damage-tolerant wings, and compensate for wing damage by adjusting their flight kinematics to produce more lift [18]. Remarkably, according to Chen’s record and our observation, 15% of honey bee workers in nature suffer from tongue damage and they can compensate for tongue structure deterioration by increasing dipping frequency to maintain a stable nectar intake rate [13]. In natural settings, honey bees can feed on a wide spectrum of food sources. However, plant secretion like nectar which is highly variable in sugar content and viscosity challenges the food intake strategy of the floral visitors (e.g., bees visit flowers producing nectar from 10–3 Pa·s to 10–1 Pa·s) [19,20,21]. Besides, depending on weather conditions, the viscosity of plant secretions may also vary during a single day [22, 23]. Conspicuously, under these natural conditions, the alteration of feeding behavior and related functional compensation in honey bees has been largely overlooked.
Therefore, in this paper, we compared morphology of the normal and damaged tongues and examined the microstructures with SEM. Also, we recorded high-speed images of both normal and partially amputated tongues while dipping artificial nectars of varying concentrations/viscosities. We obtained the energetic intake rate and quantified the effect of frequency compensation by mathematical models. Finally, among varying nectar sources, we discuss the nature-preferred nectar concentrations from the perspective of adaptation in bees with damaged tongues.
2 Materials and Methods
2.1 Preparation of Bee Samples
The honey bees were kept in two wooden beehives (100 cm × 50 cm × 50 cm) in Guangzhou, China (23.09° N, 113.30° E). They were fed with 300 mL 35% (w/w) sucrose solution, filled in a plastic box (50 cm × 10 cm × 3 cm) in the wooden beehive, 2–3 times each week. Besides, some trigs were dropped on the liquid surface of the container, on which the bees could land to ingest sucrose solution [11].
Next, we developed a method to prepare samples of living honey bees with damaged tongues. First, a worker bee was captured out of a beehive (Fig. 1a) and put into a centrifuge tube (8 mm in diameter and 40 mm in length). Then, the bee was anesthetized by placing it in a – 18 ℃ freezer (KONKA, BCD-180GY2S, China) for 150 s (Fig. 1b). Next, using scissors (ICEY, BY-3143, China), a portion of the bee’s tongue tip was amputated under a microscope (Olympus, CX33, Japan) (Fig. 1c).
Preparation of the bee samples and experimental setup to observe kinematics of damaged tongue while dipping. a A bee was first collected from a beehive, and then b anesthetized. c Tongues of the anesthetized bees were amputated by 20% and 30% respectively, and the dipping behaviors were observed by a high-speed camera connected to a microscope
2.2 Tongue Morphology
The longitudinal section of the tongue was examined by Scanning Electron Microscopy (SEM). For these observations, we prepared a total of 6 longitudinally dissected tongues from six honey bee samples. The samples were fixed in 2.5% glutaraldehyde solution for 5 h, dehydrated in a series of 50%, 70%, 80%, 90%, and 100% ethanol for 10 min each time, coated in gold–palladium (50 nm in-depth), and observed under a SEM (FEI Quanta 200, Czech Republic).
2.3 Effects of Tongue Damage on Dipping
To examine the relationship between the degree of tongue damage and the occurrence rate of dipping, we caught 35 bee workers from a beehive and subdivided them into 5 even groups randomly, namely A1, A2, A3, A4, and A5. The bees in the A1 group had intact tongues, while the bees in groups A2, A3, A4, and A5 were amputated from the tongue’s tip by 20%, 40%, 60%, and 80%, respectively. The bee’s thorax was fixed by gluing to a vertically movable positioner (Fig. 1c). This was placed under a microscope to make the bee dip the sucrose solution in a glass feeder. We recorded the full dipping process by the microscope equipped with a high-speed camera (Phantom, VR1008, USA) at a frame rate of 1000 fps [24]. The experiment was performed at 25 °C and all honey bees were provided with 35% (w/w) sucrose solution.
2.4 Dipping Sugar Water of Varying Concentrations
To observe bees with intact and damaged tongues ingesting sucrose solutions of different concentrations, we captured 75 bee workers from the beehive and subdivided them into three groups, namely B1, B2, and B3. As described earlier, the bees were put under brief anesthesia by freezing at − 18 °C for 150 s (Fig. 1b). Then, using scissors, the tongues were amputated transversely from the tongue tip by 20% and 30% in the B1 and B2 groups, respectively, while the group B3 had intact tongues as the control group. Then, in each group, bees were further subdivided into five groups to ingest 25%, 30%, 35%, 40%, and 45% (w/w) sucrose solutions, respectively. Observations on dipping cycles were made as detailed in Fig. 1c.
2.5 Dipping Sugar Water at a Constant Viscosity
To test whether the viscosity or sweetness affects the tongue damaged bee's dipping frequency, we used the inert polysaccharide Tylose to adjust the viscosity of the sucrose solutions without adding nutritional value [23]. Various amounts of Tylose HS 30000 YP2 (Shin-Etsu111 Chemical Co., Ltd., Japan) were added into 25%, 30%, 35%, and 40% sucrose solutions to maintain the viscosity at 7.2 mPa s, equal to a 45% (w/w) sucrose solution [25]. The viscosity of the solutions was measured with a digital viscometer (NDJ-5S, MINCEE, China). We then captured 75 bee workers from the beehive, which were grouped and processed according to section above and fed with 25%, 30%, 35%, 40%, and 45% sugar waters of constant viscosity, respectively.
3 Results
3.1 Tongue Morphology
In nature, honey bees use their specialized tongues to collect nectar from flowers and sap on tree bark (Fig. 2a, b). As shown in Fig. 2c, the length and the radius of a normal tongue are \(L_{{{\text{Tnor}}}} = {\text{2}}{\text{.48}} \pm {\text{0}}{\text{.03}}\;{\text{mm}}\) (n = 5 bee samples) and \(R = {\text{70}}{\text{.2}} \pm {\text{0}}{\text{.50}}\) μm (n = 5 bee samples), respectively. The tongue damage proportion is defined as \(\varsigma = {{(L_{{{\text{Tnor}}}} - L_{{\text{T}}} )} \mathord{\left/ {\vphantom {{(L_{{{\text{Tnor}}}} - L_{{\text{T}}} )} {L_{{{\text{Tnor}}}} }}} \right. \kern-\nulldelimiterspace} {L_{{{\text{Tnor}}}} }}\), in which \(L_{{\text{T}}}\) denote the lengths of damaged tongues (Fig. 2c–e). According to SEM images, the tongue sheath is comprised of ~ 120 cuticle walls connected by flexible membranes that have dense hairs distributed among them (Fig. 2c, f). The average length and radius of the tongue hair are \(h = 144.50 \pm 3.50\) μm (n = 5 bee samples) and \(r_{1} = 3.00 \pm 0.53\) μm (n = 5 bee samples), respectively. The intersegmental membranes are folded, which are prone to deformation and recovery (Fig. 2g, h) [26]. An elastic rod, connected to the tongue sheath in a compliant manner, can be driven by the base muscles to extend the intersegment membranes, and thereby erect the hair [9]. Therefore, even though the tongue tip is damaged, the erection pattern of glossal hairs still maintains.
Tongue morphology of a honey bee. Natural feeding behavior of honey bees on diverse liquid sources such as a floral nectar and b sap secreted on the tree bark. Appearance of a c normal tongue, d 20% damaged tongue, and e 30% damaged tongue while dipping nectar observed under a microscope. f–h SEM images of longitudinally dissected tongue. The tongue sheath is a thick cuticle layer, connected by flexible membranes bearing hairs
3.2 Occurrence Rate of Dipping
To examine the influence of damaged tongue on feeding, we amputate 20%, 40%, 60%, and 80% tongue length from the tongue tip and evaluate feeding behavior for 35% sucrose solution. Significantly, we find that the occurrence rate of dipping is negatively correlated with the tongue damage (Fig. 3). In normal bees, the occurrence rate of dipping is 100% (n = 7 bee samples), while the bees with 80% amputated tongue show the occurrence rate of 0% (n = 7 bee samples) (Fig. 3, video S1 in supplementary file). This indicates that severe tongue injury deprives feeding ability of honey bees, probably because of the catastrophic damage on tongue structure and neurons.
3.3 Kinematics of the Tongue Body and Hairs
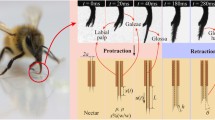
In general, up to 15% of worker bees have naturally damaged tongues corresponding to about 23% in average length from the tongue tip [13]. Considering structural damage in the natural environment, we focus on two cases of tongue damages 20% and 30%, respectively. As shown in Fig. 4a, b, dipping cycles of normal and damaged tongues exhibit a similar pattern, which can be subdivided into three stages, namely protraction, retraction, and interval with the durations of T1, T2, and T3, respectively (video S2 in supplementary file). In the protection phase, the tongue with bushy hairs extends out of the proboscis tube with galeae and labial palpi brought together (Fig. 4a, b). Then, the tongue reaches the maximum extension, followed by the tongue retraction. Notably, the tongue hairs are fully deployed and remain stable until T2 ends [27]. Currently, the hairy tongue gains the maximum diameter, namely \(D = {\text{2(}}R + h\sin \theta )\), to augment the transported fluid volume, here \(\theta\) denotes the average angle of hair erection (Fig. 4d). Lastly, the sucrose solution trapped between hair gaps is brought into the proboscis tube. In the interval stage, while the tongue remains in the tube, the sucrose solution is ingested by the cibarial pump and then is transported to the abdomen [9, 28]. However, upon tongue damage, these durations differ. In severe tongue damage, durations of T1 and T2 decline, while T3 increases (Fig. 4c, d). Specifically, in the case of bees with normal tongues while ingesting 35% sucrose solution, T1, T2, and T3 are 60 ms, 148 ms, and 50 ms, respectively. However, for bees with 20% damaged tongue to dip the same solution, T1 and T2 shorten to 45 ms and 116 ms, while T3 extends to 70 ms. Notably, the total duration of one dipping cycle is 231 ms, 27 ms shorter than the normal case, which indicates that the honeybee dips nectar faster with a damaged tongue.
Kinematics and dynamic configuration of the normal and amputated tongues. Key events of tongue kinematics in a dipping cycle of a a normal tongue and b 20% damaged tongue, respectively, while feeding on 35% sucrose solution. Tongue displacement against time in one dipping cycle of honey bees with normal (red lines), 20% damaged (blue lines), and 30% damaged (green lines) tongues for dipping c 35% and d 45% sucrose solutions, respectively. The shading indicates the error band of the experimental results
Furthermore, we plot the tongue displacement s against time considering different damage proportions for ingestion of 35% and 45% sucrose solutions (Fig. 4c, d). We notice that, for a given concentration c% of sucrose solution, the dipping duration shortens with the severity of tongue damage (Fig. 4c). Comparatively, a bee with a constant degree of tongue damage shows increased feeding duration with respect to the nectar viscosity (Fig. 4c, d). Notably, in our study, the duration of one dipping cycle in bees with \(\varsigma \;{\text{ = }}\;{\text{30\% }}\), dipping 35% sucrose solution, is 217 ± 6 ms (n = 5 bee samples), while those dipping 45% sucrose solution, have a period of 259 ± 8 ms (n = 5 bee samples), indicating an increase of 19.3% (video S3 in supplementary file).
3.4 Dipping Frequency
Next, we draw a histogram of the mean dipping frequency for a range of concentrations (varying viscosities) and tongue damage (Fig. 5a). The dipping frequency is positively correlated with the proportion of tongue damage. In normal bees, the dipping frequency is 4.24 ± 0.15 Hz for ingesting 25% sucrose solution which increased to 5.08 ± 0.18 Hz for 30% tongue damage, showing an increase of 19.8%. Notably, we observe that the dipping frequency descends with respect to the sucrose concentration (Fig. 5a). For instance, the honey bees with 20% damaged tongue, dip 25% sucrose solution at an average frequency of 4.52 ± 0.16 Hz, while it decreases to 3.46 ± 0.12 Hz for dipping 45% sucrose solution. To calculate the energetic intake rate of honey bees at different dipping frequencies, we use the experimental values from Fig. 5a to fit the dipping frequency with tongue damage proportion and sucrose concentration, formulated as
Dipping rates while imbibing artificial nectars. Honey bees with tongue damage proportions of 0%, 20%, and 30% ingesting 25%, 30%, 35%, 40%, and 45% sucrose solutions of a varying viscosities and b the constant viscosity, respectively. Statistical analysis between c the ratio of movement time to total time in one dipping cycle \(\chi\), ratio of tongue extension to retraction period \({{T_{1} } \mathord{\left/ {\vphantom {{T_{1} } {T_{2} }}} \right. \kern-\nulldelimiterspace} {T_{2} }}\), and d dipping frequency \(f\) (for constant viscosity) with tongue damage proportion \(\varsigma\), respectively. Blue squares represent the mean dipping frequency values (n = 75 dipping cycles from 25 bees), and the boxes indicate the interquartile range and whiskers represent the minimum and maximum values. The black and blue dashed lines represent the linear fitting to the experimental data. **P < 0.05 indicates the significance of the average values per section
For bees with intact tongues, the relation between dipping frequency and sucrose concentration could be fitted as
according to the data in Fig. 5a. We plot the dipping frequency of bees with varying tongue damage proportions and sucrose solutions (25%, 30%, 35%, 40%, and 45%) but adjusted to a consistent viscosity of \(\mu\) 7.2 mPa·s by adding Tylose (Fig. 5b). Notably, the frequencies of dipping nectars of a constant viscosity, are 3.47 ± 0.17 Hz for \(\varsigma = 20\%\) and 3.81 ± 0.19 Hz for \(\varsigma = {\text{3}}0\%\), respectively. This indicates that nectar viscosity is a key factor that influences the dipping frequency regardless of the nectar concentration (Fig. 5b). We define a temporal ratio of the duration of tongue movements to the total duration \(T_{{{\text{total}}}}\) of a dipping cycle as \(\chi {\kern 1pt} {\kern 1pt} = {\kern 1pt} {\kern 1pt} {{(T_{1} + T_{2} )} \mathord{\left/ {\vphantom {{(T_{1} + T_{2} )} {T_{{{\text{total}}}} }}} \right. \kern-\nulldelimiterspace} {T_{{{\text{total}}}} }}\), fitted as \(\chi {\kern 1pt} {\kern 1pt} = - {\text{0}}{\text{.582}}\varsigma + {\text{0}}{\text{.782}}\) (R2 = 0.98) (Fig. 5c). Here, the ratio expresses a declining trend with respect to tongue damage severity, which suggests bees with damaged tongues shorten dipping cycles by reducing reciprocating durations of the structurally deteriorated tongues. Thus, extending to the whole period of a feeding cycle, bees with damaged tongues exhibit a bigger dipping frequency than normal bees while ingesting the sucrose solutions of a constant viscosity of 7.2 mPa·s (Fig. 5d).
3.5 Energy Compensation for Altered Dipping Frequency
We build a model to elucidate the underlying energetic principle when the bee dips nectar with altered dipping frequency. The tongue can be regarded as a cone covered by dense hairs, surrounded by a feeding tube [9]. Considering the tongue damage, the volume of the sugar water loaded per dipping cycle can be calculated by [6, 13].
in which \(r(\varsigma )\) denotes the tongue tip radius, calculated as \(r(\varsigma ) = R\varsigma\), \(n_{{\text{h}}}\) is the tongue hair density (\(n_{{\text{h}}}\) = 2500 strand·mm−2) [9], and θtip = 1.6° ± 0.3° is the half-angle of tongue tip (Fig. 2c) (n = 5 bee samples). Then the average volumetric flux can be estimated by
Here i indicates the dipping patterns with frequency compensation (i = 1) and without frequency compensation (i = 2), respectively. The nectar density at 25 °C can be calculated as \(\rho (c) = 0.0047c + 0.9896\) [29, 30].
Then the nectar intake rate \(\dot{M}\) can be expressed by
The dynamic viscosity \(\mu (c)\) of the sucrose solution can be estimated as \(\mu (c) = {{10^{{0.8752c/(100 - c) + {{c^{2} } \mathord{\left/ {\vphantom {{c^{2} } {9901}}} \right. \kern-\nulldelimiterspace} {9901}}}} } \mathord{\left/ {\vphantom {{10^{{0.8752c/(100 - c) + {{c^{2} } \mathord{\left/ {\vphantom {{c^{2} } {9901}}} \right. \kern-\nulldelimiterspace} {9901}}}} } {10{\text{97}}}}} \right. \kern-\nulldelimiterspace} {10{\text{97}}}}\) [2, 31]. While dipping nectar, the tongue is required to overcome the viscous resistance and inertial force, with a Reynolds number \({\text{Re}} = {{\rho (c)Dv(t)} \mathord{\left/ {\vphantom {{\rho (c)Dv(t)} \mu }} \right. \kern-\nulldelimiterspace} \mu }(c)\), in which \(v(t)\) is the tongue velocity against time in one cycle and reaches the maximum in the protraction stage and can be expressed as \(v_{{\max }} = {{L_{{\text{T}}} } \mathord{\left/ {\vphantom {{L_{{\text{T}}} } {T_{1} }}} \right. \kern-\nulldelimiterspace} {T_{1} }}\). When \(\varsigma = 20\%\), the Reynolds number ranges from 9.39 to 1.95 for dipping sugar waters of 25%–45% [32, 33]. The viscous resistance at time t can be written as
in which \(k\) is the proportionality coefficient (\(k \sim 10^{{11}}\)) [31], \(D_{{\text{T}}} (t)\) denotes the diameter of the tongue with hairs against time in one cycle and \(S(t)\) is the length of tongue extending out of the proboscis tube against time in one cycle. According to these geometrical parameters, the power to overcome the viscous drag at time t can be calculated as \(P_{{\text{D}}} (t) = F_{{\text{f}}} (t)v(t) = \mu kD_{{\text{T}}} (t)S(t)S^{{\prime 2}} (t)\), in which \(S^{\prime}(t) = v(t)\). In addition, the power for tongue acceleration at time t can be written as \(P_{{\text{a}}} (t)\sim mv^{\prime}(t)v(t)\sim mS^{\prime}(t)S^{\prime\prime}(t)\). For \({{P_{{\text{a}}} (t)} \mathord{\left/ {\vphantom {{P_{{\text{a}}} (t)} {P_{{\text{D}}} }}} \right. \kern-\nulldelimiterspace} {P_{{\text{D}}} }}(t)\sim 10^{{ - 4}} \ll 1\), the effect of \(P_{{\text{a}}} (t)\) can be ignored [31, 34]. The dipping stages have a consistent ratio of T1:T2 = 2:5 (Fig. 5c) [33]. Besides, in the interval stage, the tongue stays still, therefore, the viscous resistance also becomes zero. Furthermore, the work required to resist viscous drag in one dipping cycle is \(W_{{\text{D}}} = \int_{0}^{{T_{1} + T_{2} }} {P_{{\text{D}}} } (t){\text{d}}t\). While the tongue travels through the viscous solution, the average velocity and base diameter of the tongue can be calculated as \(\bar{v}(t) = {{2L_{{\text{T}}} } \mathord{\left/ {\vphantom {{2L_{{\text{T}}} } {\left( {T_{1} (t) + T_{2} (t)} \right)}}} \right. \kern-\nulldelimiterspace} {\left( {T_{1} (t) + T_{2} (t)} \right)}}\) and \(D_{{\text{T}}} (t) = {\text{2}}R + 2h\sin \theta (t)\), respectively. Considering the frequency compensation, the average power required to resist viscous drag \(P_{{{\text{avg}}}}\) can be, therefore, expressed as
By combining Eq. (1) and Fig. 5a, we draw a surface graph to show the relationship between the dipping frequency with compensation, concentration and tongue damage proportion (Fig. 6a). The energetic intake rate is \(\dot{E}_{{{\text{com}}}} (\varsigma ) = \dot{Q}_{1} (\varsigma )\rho ec\%\), in which e = 1.54 × 104 J·g−1 is the energy content per unit mass of the solid sucrose [35]. In contrast, the energetic intake rate in the hypothetical cases with no compensation included can be written as \(\dot{E}_{{{\text{no}}}} (\varsigma ) = \dot{Q}_{2} (\varsigma )\rho ec\%\), and then the compensated portion of energetic intake rate can be stated as
Theoretical model and implications. a Dipping frequency f1 with compensation plotted against concentration c% and tongue damage proportion \(\varsigma\). b Energetic intake rate for the tongue damage and nectar concentration considering conditions with or without the dipping frequency compensation. The orange curve depicts the change of viscosity with sucrose concentration. c Lower tongue damage proportion and lower concentration increase energetic compensation rate. d The average power required to resist viscous drag against solution concentration and tongue damage proportion
The energetic intake rates of honey bees with 0%, 20%, and 30% damaged tongues dipping 25%–45% sucrose solutions are shown in Fig. 6b. The energetic intake rate is influenced by both tongue damage condition and nectar concentration. We find that the energetic intake rate reaches a maximum value, namely \(\dot{E}_{{\max }}\) = 3.43 J·s−1, in the case of normal bees having the slightest degree of tongue damage and dipping nectar at a higher concentration. Conversely, when bees with seriously damaged tongues ingest dilute sugar waters, the energetic intake rates approach lower levels (Fig. 6b, \(\dot{E}_{{\min }}\) = 2.12 J·s−1). These results indicate that the structural deterioration of the tongue can reduce the energetic intake rate, whereas ingesting more concentrated nectar with a naturally augmented dipping frequency might make up for the energetic intake loss. Furthermore, from the orange columns shown in Fig. 6b, we can see that when bees with severely damaged tongues ingest sugar water of higher concentration, \(\Delta \dot{E}(\varsigma )\) reaches a higher value, which can alleviate the loss of energetic intake rate. We then introduce an energetic compensation rate \(\alpha = {{\Delta \dot{E}(\varsigma )} \mathord{\left/ {\vphantom {{\Delta \dot{E}(\varsigma )} {(\dot{E}_{{{\text{normal}}}} - \dot{E}_{{{\text{no}}}} (\varsigma )}}} \right. \kern-\nulldelimiterspace} {(\dot{E}_{{{\text{normal}}}} - \dot{E}_{{{\text{no}}}} (\varsigma )}})\), in which \(\dot{E}_{{{\text{normal}}}}\) denotes the energetic intake rate of honey bees with normal tongues dipping various sugar waters. As \(\varsigma\) varies from 10% to 30%, \(\alpha\) drops from 83.3% to 61.6% when ingesting 25% sucrose solution (Fig. 6c). When the sucrose solution turns thicker (45% sucrose concentration), \(\alpha\) shrinks from 74.6% to 55.2% (Fig. 6c), indicating that the capacity of bees to reduce the loss of energetic intake rate due to tongue damage can be enhanced under the integrated conditions of less damaged tongue and lower nectar concentration. In other words, after the tongue loses structure, drinking diluted nectar might assist it in recovering the energetic intake rate loss compared with the full capability performed by an intact and normal tongue. Additionally, the model shows the average power required to resist viscous drag during the dipping process, which is plotted against the sucrose concentration and tongue damage degree in Fig. 6d. The maximum Pavg = 0.17 J·s−1 can be found in normal bees dipping 45% nectar. In contrast, in bees with 30% damaged tongues feeding on 25% nectar, Pavg reduces by 67% (Fig. 6d). These theoretical mappings uncover that bees with damaged tongues can reduce the average power required to resist viscous drag, which might be beneficial for continuous and effective nectar foraging.
4 Discussion
The tongue tip of a honey bee is susceptible to be damaged during foraging [13]. In this study, we elucidate the compensatory response in honey bees with damaged tongues to a variety of nectar sources in nature. We found that the dipping frequency is influenced by both damage severity of tongues and nectar viscosity. We extend our results into the following implications.
4.1 Dipping Activeness and Tongue Damage Proportion
The gustatory and mechanosensitive sensilla, distributed on the bee tongue, transmit signals to the Central Nervous System (CNS) [36,37,38,39]. This allows discriminating edible from non-edible items and, therefore, is crucial for survival [40]. In case of minor tongue damage, most sensilla are still functional to sense the sugar in the external solution and transmit signals to the CNS which activates mouthparts to complete feeding. However, upon severe tongue damage, the dipping occurrence rate gradually decreases (Fig. 3), which can be explained by two potential reasons. First, more of gustatory and mechanosensitive sensilla get demolished in tongue damage and the signals from the remnant sensilla turns ineffective for CNS to respond. Second, the motor structure of the tongue may deteriorate and the strength of the compliant link between the tongue sheath and elastic rod becomes insufficient to support tongue movement [26]. In nature, the average tongue damage proportion in bees is 23% [13], and the occurrence rate of dipping is ~ 85% (Fig. 3), which is fairly enough to satisfy the feeding needs.
4.2 Effects of Frequency Regulation
For honey bees, the structural degradation of the tongue negatively impacts the energetic intake rate while ingesting a solution of a certain concentration. However, the loss in the energetic intake rate is compensated by the altered dipping frequency. We notice that bees show a higher dipping frequency when utilizing more severely damaged tongues and dipping less concentrated nectars (Fig. 5a). After structure deterioration in the tongue, higher dipping frequency improves nectar intake rate to overcome the loss in energetic intake rate. The strategy is also applicable in the case of damaged tongue hairs [33]. In this study, we use Tylose to maintain a constant viscosity of various sucrose solutions (25%–45% w/w) (Fig. 5b). We find that the dipping frequency for the same tongue damage proportion remains consistent, suggesting that dipping frequency depends on nectar viscosity and not concentration [23, 25]. Notably, bees with damaged tongues also respond to solutions of varying viscosities, indicating that distribution of the gustatory and mechanosensitive sensilla may not be limited to the tongue tip, which certainly improves the survival in damaged tongue bees [36]. The decline in dipping frequency with an increase in viscosity is a wear-resistant behavior because higher viscosity will result in faster tongue damage [33]. There is also a friction reduction mechanism in the honey bee abdomen, and the microscale hair arrays on the surface of the posterior abdominal segment can considerably decrease wear or abrasion [41]. As shown in Fig. 3, the occurrence rate of dipping with a 60% damaged tongue is only 28.6%, suggesting severe damage in tongue tip hinders honey bee’s feeding capability. Dipping thicker nectar at a high frequency may cause catastrophic consequences in bees with damaged tongues. Moreover, upon tongue damage, the interval stage increases (Fig. 5c), suggesting a short contact time between the tongue and the nectar, which helps relieving the structural deterioration and the burden of the damaged tongue [31]. However, whether all foragers possess a regulative neural strategy to adjust the feeding frequency and time apportionment remains to be elucidated.
4.3 Theoretically Optimal Nectar Concentration
The theoretical results show that although the higher nectar concentration offers a higher energetic intake rate, it also increases the power required to resist viscous drag (Fig. 6b, d). For instance, in a case of 20% damaged tongue dipping the nectar concentrations from 20% to 50%, \(\dot{E}_{{{\text{com}}}}\) increases by 1.6 times, half of that for \(P_{{{\text{avg}}}}\). Notably, the growth in \(\dot{E}_{{{\text{com}}}}\) is not proportional to that in \(P_{{{\text{avg}}}}\), indicating that ingestion of high-concentration nectar requires highly intensive work for mismatched energy rewards, which may further aggravate the tongue damage and shorten its longevity. To explore the potential optimal concentration for the highest energetic utilization rate in the tongue damaged bees, we introduce a dimensionless parameter, namely the energetic utilization rate.
Here \(\dot{E}_{1}\) and \(P_{1}\) denote the energetic intake rate and the average power required to resist viscous drag of honey bees, respectively, for ingesting a lower concentration (c% = 20%) of nectar in nature. As shown in Fig. 7, \(\psi\) ascends against the tongue damage proportion. For instance, when bees ingest 30% sucrose solution, \(\psi\) increases from 1.06 to 1.49 as \(\varsigma\) ranges from 0 to 30%. Namely, the energetic utilization rate of bees with 30% damaged tongue is 40.6% higher than the normal bees. The principle of energetic utilization rate suggests that frequency compensation can improve \(\psi\), which may elongate the functional longevity of the tongue.
For bees with a constant tongue damage, as the concentration of sucrose solution varies from 20% to 70%, bees achieve maximum value of \(\psi\) by ingesting ~ 30% sucrose solution (Fig. 7). While, with \(\varsigma\) ranging from 0 to 36%, the optimal concentration shifts from 27% to 31%, which is slightly lower than the real concentration of 35% in nature [19]. The discrepancy between the theoretical results and natural floral nectar concentrations may be interpreted from the perspective of ecological requirements. In nature, the temperature inside flowers (26–36 °C) is a bit higher than the laboratory settings (25 °C), which lowers nectar viscosity, making it easier to drink as a thermal reward [22, 23]. Due to the inevitable tongue damage, honey bees have to meet the contradictive demands of reducing energetic intake rate loss and relieving the burden of damaged tongue. By regulating dipping frequency, the energetic utilization rate augments and both demands could be satisfied. Meanwhile, for tongue damaged bees, the optimal nectar concentration changes only by 4%. Therefore, bees may not need to switch the nectariferous sources for a higher energetic intake rate, assuring their survival.
5 Conclusion
In this study, using a combination of biological experiments and mathematical modeling, we reveal the compensation behavior in honey bees having different proportions of tongue damages ingesting a range of concentrated/viscous nectars. The frequency compensation reduces the loss in energetic intake rate and improves the energetic utilization rate. Furthermore, we provide a mechanistic explanation for the preference for ~ 30% nectar, from the perspective of tongue dynamics. We show that the theoretically optimal concentration is little influenced by the tongue damage because of the effects of frequency regulation. However, behavioral consequences related to alterations induced by tongue damage remain to be further studied at the neuromodulator level. This study broadens our knowledge about the compensation mechanisms in honey bees and sheds light on potential applications in reducing functional degradation in man-made micromechanics such as microfluidic pumps.
References
Reis, P. M., Jung, S., Aristoff, J. M., & Stocker, R. (2010). How cats lap: Water uptake by Felis catus. Science, 330, 1231–1234.
Kim, W., & Bush, J. W. M. (2012). Natural drinking strategies. Journal of Fluid Mechanics, 705, 7–25.
Lehnert, M. S., Mulvane, C. P., & Brothers, A. (2014). Mouthpart separation does not impede butterfly feeding. Arthropod Structure and Development, 43, 97–102.
Gart, S., Socha, J. J., Vlachos, P. P., & Jung, S. (2015). Dogs lap using acceleration-driven open pumping. Proceedings of the National Academy of Sciences, 112, 15798–15802.
Lee, S. J., Kim, B. H., & Lee, J. Y. (2009). Experimental study on the fluid mechanics of blood sucking in the proboscis of a female mosquito. Journal of Biomechanics, 42, 857–864.
Yang, H., Wu, J. N., & Yan, S. Z. (2014). Effects of erectable glossal hairs on a honeybee’s nectar-drinking strategy. Applied Physics Letters, 104, 263701.
Wu, J. N., Zhu, R. G., Yan, S. Z., & Yang, Y. Q. (2015). Erection pattern and section-wise wettability of honeybee glossal hairs in nectar feeding. Journal of Experimental Biology, 218, 664–667.
Krenn, H. W., Plant, J., & Szucsich, N. U. (2005). Mouthparts of flower-visiting insects. Arthropod Structure and Development, 34, 1–40.
Zhao, J. L., Wu, J. N., & Yan, S. Z. (2015). Erection mechanism of glossal hairs during honeybee feeding. Journal of Theoretical Biology, 386, 62–68.
Tautz, J. (2008). The Buzz about Bees: Biology of a Superorganism. Springer Science & Business Media.
Zhu, R. G., Lv, H. S., Liu, T. Y., Yang, Y. Q., Wu, J. N., & Yan, S. Z. (2016). Feeding kinematics and nectar intake of the honey bee tongue. Journal of Insect Behavior, 29, 325–339.
Pflumm, W. (1985). Influence of nectar-supply rate on the number of flowers visited by a honeybee on each collecting flight. Oecologia, 66, 207–210.
Chen, Y., Wu, J. N., Yang, Y. Q., & Yan, S. Z. (2020). Functional compensation in a honey bee’s damaged tongue while dipping nectar. Journal of Insect Behavior, 33, 71–82.
Pape-Lindstrom, P., Feller, R., Stancyk, S., & Woodin, S. (1997). Sublethal predation: Field measurements of arm tissue loss from the ophiuroid Microphiopholisgracillima and immunochemical identification of its predators in North Inlet, South Carolina, USA. Marine Ecology Progress Series, 156, 131–140.
Tomiyama, T., & Ito, K. (2006). Regeneration of lost siphon tissues in the tellinacean bivalve Nuttallia olivacea. Journal of Experimental Marine Biology and Ecology, 335, 104–113.
Lindsay, S. M. (2010). Frequency of injury and the ecology of regeneration in marine benthic invertebrates. Integrative and Comparative Biology, 50, 479–493.
Abrams, M. J., Basinger, T., Yuan, W., Guo, C.-L., & Goentoro, L. (2015). Self-repairing symmetry in jellyfish through mechanically driven reorganization. Proceedings of the National Academy of Sciences, 112, 3365–3373.
Rajabi, H., Dirks, J.H., & Gorb, S. N. (2020). Insect wing damage: Causes, consequences and compensatory mechanisms. The Journal of Experimental Biology, 223, jeb215194.
Pyke, G. H., & Waser, N. M. (1981). The production of dilute nectars by hummingbird and honeyeater flowers. Biotropica, 13, 260–270.
Nardone, E., Dey, T., & Kevan, P. G. (2013). The effect of sugar solution type, sugar concentration and viscosity on the imbibition and energy intake rate of bumblebees. Journal of Insect Physiology, 59, 919–933.
Lechantre, A., Michez, D., & Damman, P. (2019). Collection of nectar by bumblebees: How the physics of fluid demonstrates the prominent role of the tongue’s morphology. Soft Matter, 15, 6392–6399.
Telis, V. R. N., Telis-Romero, J., Mazzotti, H., & Gabas, A. L. (2007). Viscosity of aqueous carbohydrate solutions at different temperatures and concentrations. International Journal of Food Properties, 10, 185–195.
Nicolson, S. W., Veer, L. D., Köhler, A., & Pirk, C. W. W. (2013). Honeybees prefer warmer nectar and less viscous nectar, regardless of sugar concentration. Proceedings of the Royal Society B: Biological Sciences, 280, 20131597.
Liao, C. Y., Xu, Y. J., Sun, Y., Lehnert, M. S., Xiang, W. W. K., Wu, J. N., & Wu, Z. G. (2020). Feeding behavior of honey bees on dry sugar. Journal of Insect Physiology, 124, 104059.
Shi, L. H., Nicolson, S. W., Yang, Y. Q., Wu, J. N., Yan, S. Z., & Wu, Z. G. (2020). Drinking made easier: Honey bee tongues dip faster into warmer and/or less viscous artificial nectar. Journal of Experimental Biology, 223, jeb229799.
Yang, Y. Q., Wu, J. N., Zhu, R. G., Li, C. C., & Yan, S. Z. (2017). The honeybee’s protrusible glossa is a compliant mechanism. Journal of Bionic Engineering, 14, 607–615.
Lechantre, A., Draux, A., Hua, H.-A.B., Michez, D., Damman, P., & Brau, F. (2021). Essential role of papillae flexibility in nectar capture by bees. Proceedings of the National Academy of Sciences, 118, e2025513118.
Zhao, J. L., Meng, F. Y., Yan, S. Z., Wu, J. N., Liang, Y. J., & Zhang, Y. L. (2019). Abdominal pumping involvement in the liquid feeding of honeybee. Journal of Insect Physiology, 112, 109–116.
Pivnick, K. A., & McNeil, J. N. (1985). Effects of nectar concentration on butterfly feeding: Measured feeding rates for Thymelicus lineola (Lepidoptera: Hesperiidae) and a general feeding model for adult Lepidoptera. Oecologia, 66, 226–237.
Shi, L. H., Wu, J. N., Krenn, H. W., Yang, Y. Q., & Yan, S. Z. (2019). Temporal model of fluid-feeding mechanisms in a long proboscid orchid bee compared to the short proboscid honey bee. Journal of Theoretical Biology, 484, 110017.
Wu, J. N., Shi, G. Y., Zhao, Y. W., & Yan, S. Z. (2018). How to dip nectar: Optimal time apportionment in natural viscous fluid transport. Journal of Physics D: Applied Physics, 51, 245401.
Pan, Z., Whitehead, J., Thomson, S., & Truscott, T. (2016). Error propagation dynamics of PIV-based pressure field calculations: How well does the pressure Poisson solver perform inherently? Measurement Science and Technology, 27, 84012–84012.
Wu, J. N., Chen, Y., Li, C. C., Lehnert, M. S., Yang, Y. Q., & Yan, S. Z. (2019). A quick tongue: Older honey bees dip nectar faster to compensate for mouthpart structure deterioration. Journal of Experimental Biology, 222, jeb.212191.
Kim, W., Gilet, T., & Bush, J. W. M. (2011). Optimal concentrations in nectar feeding. Proceedings of the National Academy of Sciences, 108, 16618–16621.
Daniel, T. L., Kingsolver, J. G., & Meyhöfer, E. (1989). Mechanical determinants of nectar-feeding energetics in butterflies: Muscle mechanics, feeding geometry, and functional equivalence. Oecologia, 79, 66–75.
Kumar, R., & Kumar, N. R. (2016). Scanning electron microscopic study on the mouthparts of Apis florea (Hymenoptera: Apidae). Apidologie, 47, 717–727.
Gronenberg, W., & Couvillon, M. J. (2010). Brain composition and olfactory learning in honey bees. Neurobiology of Learning and Memory, 93, 435–443.
Farina, W. M., & Núñez, J. A. (1991). Trophallaxis in the honeybee, Apis mellifera (L.) as related to the profitability of food sources. Animal Behaviour, 42, 389–394.
Whitehead, A., & Larsen, J. (1976). Ultrastructure of the contact chemoreceptors of Apis mellifera L. (Hymenoptera: Apidae). International Journal of Insect Morphology and Embryology, 5, 301–315.
de Brito, S. M. G. (2011). Taste perception in honey bees. Chemical Senses, 36, 675–692.
Wang, M. Y., Chen, W. H., Zhao, J. L., Yu, L., & Yan, S. Z. (2021). Hairy-layer friction reduction mechanism in the honeybee abdomen. ACS Applied Materials and Interfaces, 13, 24524–24531.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 51905556) and the research grant of Sun Yat-Sen University for Bairen Plan (Grant no. 76200-18841223).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of Interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 349 KB)
Supplementary file2 (MP4 569 KB)
Supplementary file3 (MP4 382 KB)
Rights and permissions
About this article
Cite this article
Wang, J., Sun, Y., Zhang, W. et al. Effects of Nectar Property on Compensated Dipping Behavior of Honey Bees with Damaged Tongues. J Bionic Eng 18, 1225–1234 (2021). https://doi.org/10.1007/s42235-021-00094-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42235-021-00094-9