Abstract
In this study, magnesia–nickel silicate ore was investigated, which contained 0.83% Ni and antigorite, chlorite, magnetite (Fe3O4), quartz, and mica as the main minerals. Chloride roasting and magnetic separation were used to treat the ore. The addition of red mud enhanced the transformation of nickel silicate to a strongly magnetic mineral mainly comprising metallic Ni and that of hematite to Fe3O4 and metallic Fe. Test results showed that a Ni concentrate with a Ni content of 11.41% and Ni recovery of 92.77% was achieved under the following optimal conditions: roasting temperature: 1373.15 K; roasting time: 120 min; CaCl2 dosage: 20%; coke dosage: 14%; red mud dosage: 30%; magnetic field intensity = 0.14 T; and grinding fineness: 90% < 0.05 mm. The major minerals in the Ni concentrate were Fe3O4, metallic Fe, and metallic Ni. Sc entered the magnetic separation tailings, creating a favorable condition for the further extraction of Sc by hydrometallurgy. The thermodynamic calculation results are in good agreement with the test results.
Graphical Abstract










Similar content being viewed by others
References
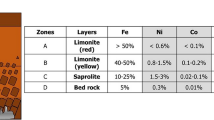
Kee-seok K, In-kook B, Hyung-Seok K (2016) A study on classification of limonite and saprolite from nickel laterite ores. J Korean Inst Resour Recycl 25:40–47. https://doi.org/10.7844/kirr.2016.25.1.40
Butt CRM, Cluzel D (2013) Nickel laterite ore deposits: weathered serpentinites. Elements 9:123–128. https://doi.org/10.2113/gselements.9.2.123
Tupaz CAJ, Watanabe Y, Sanematsu K, Echigo T (2020) Mineralogy and geochemistry of the Berong Ni-Co laterite deposit, Palawan, Philippines. J. Ore Geol Rev 125:103686. https://doi.org/10.1016/j.oregeorev.2020.103686
Zhao D, Ma BZ, Shi BD, Zhou ZE, Xing P, Wang CY (2020) Mineralogical characterization of limonitic laterite from Africa and its proposed processing route. J Sustain Metall 6:491–503. https://doi.org/10.1007/s40831-020-00290-7
Meshram P (2019) Abhilash, Pandey BD. Miner Process Extr Metall Rev 40:157–193. https://doi.org/10.1080/08827508.2018.1514300
Xiao JH, Ding W, Peng Y, Chen T, Zou K, Wang Z (2020) Extraction of Nickel from Garnierite Laterite Ore Using Roasting and Magnetic Separation with Calcium Chloride and Iron Concentrate. Minerals 10:352. https://doi.org/10.3390/min10040352
Zhu DQ, Cui Y, Hapugoda S, Vining K, Pan J (2012) Mineralogy and crystal chemistry of a low grade nickel laterite ore. Trans Nonferrous Met Soc China 22:907–916. https://doi.org/10.1016/S1003-6326(11)61264-8
Sarbishei S, Tafaghodi Khajavi LT (2020) The effect of sulfur content of rotary kiln fuel on the composition of nickel laterite calcine. Fuel 280:118648. https://doi.org/10.1016/j.fuel.2020.118648
Villanova-de-Benavent C, Domènech C, Tauler E, Galí S, Tassara S, Proenza JA (2017) Fe-Ni-bearing serpentines from the saprolite horizon of Caribbean Ni-laterite deposits: new insights from thermodynamic calculations. Mineral Deposita 52:979–992. https://doi.org/10.1007/s00126-016-0683-7
Zappala LC, Balucan RD, Vaughan J, Steel KM (2020) Development of a nickel extraction-mineral carbonation process: analysis of leaching mechanisms using regenerated acid. Hydrometallurgy 197:105482. https://doi.org/10.1016/j.hydromet.2020.105482
Liu HY, Xiang N, Shen XY, Zhai YC, Han C (2020) Decrease of material burden in a novel alkali-saving reduction treatment process of nickel slag based on NaOH roasting. JOM 72:2686–2696. https://doi.org/10.1007/s11837-019-03914-w
Tian HY, Pan J, Zhu DQ, Yang CC, Guo ZQ, Xue YX (2020) Improved beneficiation of nickel and iron from a low-grade saprolite laterite by addition of limonitic laterite ore and CaCO3. J Mater Res Technol 9:2578–2589. https://doi.org/10.1016/j.jmrt.2019.12.088
Zhu DQ, Pan LT, Guo ZQ, Pan J, Zhang F (2019) Utilization of limonitic nickel laterite to produce ferronickel concentrate by the selective reduction-magnetic separation process. Adv Powder Technol 30:451–460. https://doi.org/10.1016/j.apt.2018.11.024
Tsuji H (2012) Behavior of reduction and growth of metal in smelting of saprolite Ni-ore in a rotary kiln for production of ferro-nickel alloy. ISIJ Int 52:1000–1009. https://doi.org/10.2355/isijinternational.52.1000
Cui FH, Mu WN, Zhai YC, Guo XY (2020) The selective chlorination of nickel and copper from low-grade nickel-copper sulfide–oxide ore: Mechanism and kinetics. Sep Purif Technol 239:116577. https://doi.org/10.1016/j.seppur.2020.116577
Yang J, Zhang GQ, Ostrovski O, Jahanshahi S (2019) Selective reduction of an Australian garnieritic laterite ore. Miner Eng 131:79–89. https://doi.org/10.1016/j.mineng.2018.10.018
Xiao JH, Zhang YS (2020) Extraction of cobalt and iron from refractory Co-bearing sulfur concentrate. Processes 8:200. https://doi.org/10.3390/pr8020200
Li B, Ding ZG, Wei YG, Zhou SW, Wang H (2018) Reduction of nickel and iron from low-grade nickel laterite ore via a solid-state deoxidization method using methane. Mater Trans 59:1180–1185. https://doi.org/10.2320/matertrans.M2017351
Ma BZ, Yang WJ, Xing P, Wang CY, Chen YQ, Lv DY (2017) Pilot-scale plant study on solid-state metalized reduction-magnetic separation for magnesium-rich nickel oxide ores. Int J Miner Process 169:99–105. https://doi.org/10.1016/j.minpro.2017.11.002
Ribeiro PPM, Neumann R, dos Santos IDd, Rezende MC, Radino-Rouse P, Dutra AJB (2019) Nickel carriers in laterite ores and their influence on the mechanism of nickel extraction by sulfation-roasting-leaching process. Miner Eng 131:90–97. https://doi.org/10.1016/j.mineng.2018.10.022
Adeela N, Khan U, Naz S, Khan K, Sagar RUR, Aslam S, Wu D (2018) Role of Ni concentration on structural and magnetic properties of inverse spinel ferrite. Mater Res Bull 107:60–65. https://doi.org/10.1016/j.materresbull.2018.06.032
Kolmachikhina OB, Chunarev AA, Naboichenko SS (2015) Preparation of oxidized nickel ores for hydrometallurgical processing. Metallurgist 59:727–732. https://doi.org/10.1007/s11015-015-0166-6
Ding W, Xiao JH, Peng Y, Shen SY, Chen T, Zou K, Wang Z (2020) A novel process for extraction of iron from a refractory red mud. Physicochem Probl Miner Process 56:125–136. https://doi.org/10.37190/ppmp/127319
Hang GH, Xue ZL, Wang JH, Wu YJ (2020) Mechanism of calcium–sulphate on the aggregation and growth of ferronickel particles in the self-reduction of saprolitic nickel laterite ore. Metals 10:423. https://doi.org/10.3390/met10040423
Archambo M, Kawatra SK (2020) Red mud: fundamentals and new avenues for utilization. Process Extr Metall Rev, Miner. https://doi.org/10.1080/08827508.2020.1781109
Paramguru RK, Rath PC, Misra VN (2004) Trends in red mud utilization: a review. Miner Process Extr Metall Rev 26(1):1–29. https://doi.org/10.1080/08827500490477603
Yu JW, Han YX, Li YJ, Gao P (2020) Recent advances in magnetization roasting of refractory iron ores: a technological review in the past decade. Miner Process Extr Metall Rev 41:349–359. https://doi.org/10.1080/08827508.2019.1634565
Mishra S (2020) Review on reduction kinetics of iron ore–coal composite pellet in alternative and sustainable ironmaking. J Sustain Metall 6:541–556. https://doi.org/10.1007/s40831-020-00299-y
Chen D, Zhu DQ, Hong L, Chen Y, Xu JF, Wu L (2015) Preparation of pre-reduced pellet using pyrite cinder containing nonferrous metals with high temperature chloridizing reduction roasting technology: effect of CaCl2 additive. J Cent South Univ 22:4154–4161. https://doi.org/10.1007/s11771-015-2962-3
Matus C, Stopic S, Etzold S, Kremer D, Wotruba H, Dertmann C, Telle R, Friedrich B, Knops P (2020) Mechanism of nickel, magnesium, and iron recovery from olivine bearing ore during leaching with hydrochloric acid including a carbonation pre-treatment. Metals 10:811. https://doi.org/10.3390/met10060811
Li S, Kang Z, Liu W, Lian YC, Yang HS (2021) Reduction behavior and direct reduction kinetics of red mud-biomass composite pellets. J Sustain Metall 7:126–135. https://doi.org/10.1007/s40831-020-00326-y
Pintowantoro S, Widyartha AB, Setiyorini Y, Setiyorini Y, Abdul F (2021) Sodium thiosulfate and natural sulfur: novel potential additives for selective reduction of limonitic laterite ore. J Sustain Metall. https://doi.org/10.1007/s40831-021-00352-4
Acknowledgements
This work was funded by the Research Fund Program of Innovation Center of Rare Earth Resources Development and Utilization, China Geological Survey (Grant No. 2021XTZX01), the Sichuan Science and Technology Program (Grant Nos. 2021YJ0057, 2021YFG0268, 2019FS0451, and 2019FS0452), Key Laboratory of Guangdong Provincial Key Laboratory of Radioactive and Rare Resource Utilization (Grant No. 2018B030322009), and the Research Fund Program of Key Laboratory of Sichuan Province for Comprehensive Utilization of Vanadium and Titanium Resources Foundation (Grant No. 2018FTSZ35).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Adam Clayton Powell.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiao, J., Xiong, W., Zou, K. et al. Extraction of Nickel from Magnesia–Nickel Silicate Ore. J. Sustain. Metall. 7, 642–652 (2021). https://doi.org/10.1007/s40831-021-00364-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00364-0