Abstract
Purpose of Review
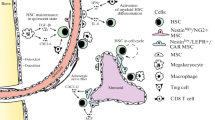
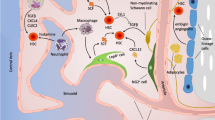
Hematopoietic stem and progenitor cells (HSC/HSPCs) originate in bone marrow (BM) niches and facilitate immune cell production throughout life. Critical signals from physical and soluble factors in the niche, including the native low oxygen (“hypoxic”) environment, regulate the phenotype, function, and cell fate decisions of HSCs. In this review, we highlight foundational knowledge, recent insights detailing the implications of varied oxygen levels in the BM niche and how manipulation of the oxygen environment in experimental conditions impact the data/interpretation of HSC/HSPC populations.
Recent Findings
Using novel techniques, characterization of oxygen tension in distinct BM niches has shaped foundational studies of HSC/HSPC localization and biology. Additional studies utilizing sorted, live HSCs that remain in their physiologic low oxygen environment, rather than exposing them to air and placing them back into low oxygen conditions, continue to advance our knowledge of endogenous HSC phenotype, heterogeneity, signaling, and function/fate decisions.
Summary
The low oxygen (O2) environment of the BM niche plays a vital role in normal and malignant hematopoiesis including HSC/HSPC metabolism, signaling/regulatory mechanisms, quiescence, stem cell fate/differentiation, engraftment/transplantation ability, and therapeutic response. Technological innovations have advanced our current understanding of HSC localization and regulation within the BM niche with respect to the low O2 environment. Initial studies identified unique oxygen gradients as well as loss of HSC after removal from low O2 (i.e., exposure to room air using whole BM). Recent studies being performed with sorted, live HSC/HSPC populations remaining in their native low O2 environment have provided novel insight, direct comparisons, and identification of unique HSC/HSPC subpopulations demonstrating alterations in signaling, heterogeneity, cell fidelity, and enhancement in the HSC/HSPC phenotype/function not identified in previous studies. Further studies of hematopoietic cell subpopulations in their native low O2 environment will shed additional light on endogenous HSC/HSPC biology leading to translational implications for transplantation and therapeutic intervention.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150–61.
Spencer JA, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–73.
Scadden DT. Nice neighborhood: emerging concepts of the stem cell niche. Cell. 2014;157(1):41–50.
Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91(3):335–44.
Lord BI, Testa NG, Hendry JH. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood. 1975;46(1):65–72.
Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25.
May M, Slaughter A, Lucas D. Dynamic regulation of hematopoietic stem cells by bone marrow niches. Curr Stem Cell Rep. 2018;4(3):201–8.
Mantel CR, et al. Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell. 2015;161(7):1553–65.
Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20(5):303-320. This review comprehensively covers our current knowledge on HSC activity and their interaction with other supporting cells within the BM niche.
Tikhonova AN, et al. The bone marrow microenvironment at single-cell resolution. Nature. 2019;569(7755):222-228. This study maps the BM vasculature, perivasculature, and osteoblast niche at a single-cell resolution in not only homeostasis but also stree hematopoiesis.
Umemoto T, et al. Ca(2+)-mitochondria axis drives cell division in hematopoietic stem cells. J Exp Med. 2018;215(8):2097-2113. This study shows quiescent HSCs demonstrate low mitochondrial membrane potential.
Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–34.
Wei Q, Frenette PS. Niches for hematopoietic stem cells and their progeny. Immunity. 2018;48(4):632–48.
Christodoulou C, et al. Live-animal imaging of native haematopoietic stem and progenitor cells. Nature. 2020;578(7794):278-283. This study demonstrates HSCs reside in approximately 3% O2 tension in the BM niche.
Parmar K, et al. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104(13):5431–6.
Roy S, et al. Hypoxia improves expansion potential of human cord blood-derived hematopoietic stem cells and marrow repopulation efficiency. Eur J Haematol. 2012;88(5):396–405.
Ivanović Z, et al. Incubation of murine bone marrow cells in hypoxia ensures the maintenance of marrow-repopulating ability together with the expansion of committed progenitors. Br J Haematol. 2000;108(2):424–9.
Paredes-Gamero EJ, Barbosa CM, Ferreira AT. Calcium signaling as a regulator of hematopoiesis. Front Biosci (Elite Ed). 2012;4:1375–84.
Luchsinger LL, et al. Harnessing hematopoietic stem cell low intracellular calcium improves their maintenance in vitro. Cell Stem Cell. 2019;25(2):225-240.e7 This article is the first to identify the importance of intracellular calcium levels in HSC maintenance.
Broxmeyer HE, et al. Numbers of long-term hematopoietic stem cells from bone marrow of fanca and fancc knockout mice can be greatly enhanced by their collection and processing in physioxia conditions. Blood Cells Mol Dis. 2021;86:102492.
Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–16.
Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97(8):2293–9.
Quiñones-Hinojosa A, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494(3):415–34.
Itkin T, et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;532(7599):323–8.
Schoeters GE, Vanderboroght OL. Haemopoietic stem cell concentration and CFUs in DNA synthesis in bone marrow from different bone regions. Experientia. 1980;36(4):459–61.
Eliasson P, et al. Hypoxia mediates low cell-cycle activity and increases the proportion of long-term-reconstituting hematopoietic stem cells during in vitro culture. Exp Hematol. 2010;38(4):301-310.e2.
Harrison JS, et al. Oxygen saturation in the bone marrow of healthy volunteers, in Blood. United States. 2002;p. 394.
Mas-Bargues C, et al. Relevance of oxygen concentration in stem cell culture for regenerative medicine. Int J Mol Sci. 2019;20(5).
Ceradini DJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10(8):858–64.
Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611.
Kunisaki Y, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–43.
Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5.
Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–21.
Winkler IG, et al. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116(3):375–85.
Telford WG, et al. Side population analysis using a violet-excited cell-permeable DNA binding dye. Stem Cells. 2007;25(4):1029–36.
Lo Celso C, Lin CP, Scadden DT. In vivo imaging of transplanted hematopoietic stem and progenitor cells in mouse calvarium bone marrow. Nat Protoc. 2011;6(1):1–14.
Christodoulou, C., et al., Live-animal imaging of native haematopoietic stem and progenitor cells. Nature, 2020. 578(7794): p. 278-283.
Turcotte R, et al. Image-guided transplantation of single cells in the bone marrow of live animals. Sci Rep. 2017;7(1):3875.
Nombela-Arrieta C, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15(5):533–43.
Huang X, et al. Hypoxia signaling pathway in stem cell regulation: good and evil. Curr Stem Cell Rep. 2018;4(2):149–57.
Broxmeyer HE, et al. The importance of hypoxia and extra physiologic oxygen shock/stress for collection and processing of stem and progenitor cells to understand true physiology/pathology of these cells ex vivo. Curr Opin Hematol. 2015;22(4):273–8.
Lin Q, et al. Oxygen and cell fate decisions. Gene Regul Syst Bio. 2008;2:43–51.
Chandel NS, et al. Metabolic regulation of stem cell function in tissue homeostasis and organismal ageing. Nat Cell Biol. 2016;18(8):823–32.
Adelman DM, Maltepe E, Simon MC. Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes Dev. 1999;13(19):2478–83.
Silberstein L, et al. Proximity-based differential single-cell analysis of the niche to identify stem/progenitor cell regulators. Cell Stem Cell. 2016;19(4):530–43.
Lassailly F, et al. Multimodal imaging reveals structural and functional heterogeneity in different bone marrow compartments: functional implications on hematopoietic stem cells. Blood. 2013;122(10):1730–40.
Carrelha J, et al. Hierarchically related lineage-restricted fates of multipotent haematopoietic stem cells. Nature. 2018;554(7690):106–11.
Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2(3):336–61.
Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors–similar but not identical. Mol Cells. 2010;29(5):435–42.
Guitart AV, et al. Hif-2α is not essential for cell-autonomous hematopoietic stem cell maintenance. Blood. 2013;122(10):1741–5.
Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol. 2011;76:347–53.
Takubo K, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7(3):391–402.
Simsek T, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7(3):380–90.
Kocabas F, et al. Hypoxic metabolism in human hematopoietic stem cells. Cell Biosci. 2015;5:39.
Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007(407):cm8.
Kierans SJ, Taylor CT. Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J Physiol. 2021;599(1):23–37.
Carmeliet P, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394(6692):485–90.
Yoon D, et al. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem. 2006;281(35):25703–11.
Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378–89.
Korovina I, et al. Hematopoietic hypoxia-inducible factor 2α deficiency ameliorates pathological retinal neovascularization via modulation of endothelial cell apoptosis. Faseb j. 2019;33(2):1758–70.
Rouault-Pierre K, et al. HIF-2α protects human hematopoietic stem/progenitors and acute myeloid leukemic cells from apoptosis induced by endoplasmic reticulum stress. Cell Stem Cell. 2013;13(5):549–63.
Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110(8):3056–63.
Madreiter-Sokolowski CT, Thomas C, Ristow M. Interrelation between ROS and Ca(2+) in aging and age-related diseases. Redox Biol. 2020;36:101678.
Delierneux C, et al. Mitochondrial calcium regulation of redox signaling in cancer. Cells. 2020;9(2).
Adams GB, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603.
Seagroves TN, et al. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol Cell Biol. 2001;21(10):3436–44.
Papandreou I, et al. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3(3):187–97.
Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9(4):298–310.
Kohli L, Passegué E. Surviving change: the metabolic journey of hematopoietic stem cells. Trends Cell Biol. 2014;24(8):479–87.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33.
Passaro D, et al. Increased vascular permeability in the bone marrow microenvironment contributes to disease progression and drug response in acute myeloid leukemia. Cancer Cell. 2017;32(3):324-341.e6.
Batsivari, A., et al., Author Correction: Dynamic responses of the haematopoietic stem cell niche to diverse stresses, in Nat Cell Biol. 2020: England. p. 257.
Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114(6):1150–7.
Kusumbe AP, et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. 2016;532(7599):380–4.
Kovtonyuk LV, et al. Inflamm-aging of hematopoiesis, hematopoietic stem cells, and the bone marrow microenvironment. Front Immunol. 2016;7:502.
Steensma DP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9–16.
Mosteo L, et al. The dynamic interface between the bone marrow vascular niche and hematopoietic stem cells in myeloid malignancy. Front Cell Dev Biol. 2021;9:635189.
Park DS, et al. Clonal hematopoiesis of indeterminate potential and its impact on patient trajectories after stem cell transplantation. PLoS Comput Biol. 2019;15(4):e1006913.
Vas V, et al. Aging of the microenvironment influences clonality in hematopoiesis. PLoS One. 2012;7(8):e42080.
Masala E, et al. Severe hypoxia selects hematopoietic progenitors with stem cell potential from primary myelodysplastic syndrome bone marrow cell cultures. Oncotarget. 2018;9(12):10561–71.
Mimeault M, Batra SK. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J Cell Mol Med. 2013;17(1):30–54.
Zhe N, et al. Heme oxygenase-1 plays a crucial role in chemoresistance in acute myeloid leukemia. Hematology. 2015;20(7):384–91.
Lv X, et al. The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism. Genes Dis. 2017;4(1):19–24.
Wang Y, et al. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8(4):399–411.
Zhang H, et al. HIF1α is required for survival maintenance of chronic myeloid leukemia stem cells. Blood. 2012;119(11):2595–607.
Tong H, et al. Hypoxia-inducible factor-1α expression indicates poor prognosis in myelodysplastic syndromes. Leuk Lymphoma. 2012;53(12):2412–8.
Wellmann S, et al. Activation of the HIF pathway in childhood ALL, prognostic implications of VEGF. Leukemia. 2004;18(5):926–33.
Maxwell PH, et al. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1997;94(15):8104–9.
Vukovic M, et al. Hif-1α and Hif-2α synergize to suppress AML development but are dispensable for disease maintenance. J Exp Med. 2015;212(13):2223–34.
Velasco-Hernandez T, et al. HIF-1α can act as a tumor suppressor gene in murine acute myeloid leukemia. Blood. 2014;124(24):3597–607.
Méndez-Ferrer S, et al. Bone marrow niches in haematological malignancies. Nat Rev Cancer. 2020;20(5):285–98.
Skrede S, Iversen PO. Enhanced oxygen consumption and fatty acid metabolism in rat bone marrow with acute promyelocytic leukaemia. Leuk Res. 1995;19(7):463–7.
Koczula KM, et al. Metabolic plasticity in CLL: adaptation to the hypoxic niche. Leukemia. 2016;30(1):65–73.
Dias S, et al. Inhibition of both paracrine and autocrine VEGF/ VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci U S A. 2001;98(19):10857–62.
Yao Y, et al. Leukemia stem cell-bone marrow microenvironment interplay in acute myeloid leukemia development. Exp Hematol Oncol. 2021;10(1):39.
Karp JE, et al. Targeting vascular endothelial growth factor for relapsed and refractory adult acute myelogenous leukemias: therapy with sequential 1-beta-d-arabinofuranosylcytosine, mitoxantrone, and bevacizumab. Clin Cancer Res. 2004;10(11):3577–85.
Ossenkoppele GJ, et al. Addition of bevacizumab to chemotherapy in acute myeloid leukemia at older age: a randomized phase 2 trial of the Dutch-Belgian Cooperative Trial Group for Hemato-Oncology (HOVON) and the Swiss Group for Clinical Cancer Research (SAKK). Blood. 2012;120(24):4706–11.
Zahiragic L, et al. Bevacizumab reduces VEGF expression in patients with relapsed and refractory acute myeloid leukemia without clinical antileukemic activity, in Leukemia. England. 2007;1310–2.
Fiedler W, et al. A phase 2 clinical study of SU5416 in patients with refractory acute myeloid leukemia. Blood. 2003;102(8):2763–7.
Fiedler W, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105(3):986–93.
Stopeck A, et al. Results of a Phase I dose-escalating study of the antiangiogenic agent, SU5416, in patients with advanced malignancies. Clin Cancer Res. 2002;8(9):2798–805.
Sukbuntherng J, et al. Pharmacokinetics and interspecies scaling of a novel VEGF receptor inhibitor, SU5416. J Pharm Pharmacol. 2001;53(12):1629–36.
Fiedler W, et al. A phase I/II study of sunitinib and intensive chemotherapy in patients over 60 years of age with acute myeloid leukaemia and activating FLT3 mutations. Br J Haematol. 2015;169(5):694–700.
Debant M, et al. Calcium signaling and cell fate: how can Ca2+ signals contribute to wrong decisions for Chronic Lymphocytic Leukemic B lymphocyte outcome? Int J Dev Biol. 2015;59(7–9):379–89.
Ciarcia R, et al. Dysregulated calcium homeostasis and oxidative stress in chronic myeloid leukemia (CML) cells. J Cell Physiol. 2010;224(2):443–53.
Uslu M, Albayrak E, Kocabaş F. Temporal modulation of calcium sensing in hematopoietic stem cells is crucial for proper stem cell expansion and engraftment. J Cell Physiol. 2020;235(12):9644–66.
Kindwall-Keller TL, Ballen KK. Umbilical cord blood: the promise and the uncertainty. Stem Cells Transl Med. 2020;9(10):1153–62.
Kindwall-Keller TL, et al. Prospective study of one- vs two-unit umbilical cord blood transplantation following reduced intensity conditioning in adults with hematological malignancies. Bone Marrow Transplant. 2012;47(7):924–33.
Gutman JA, et al. Double unit cord blood transplantation: who wins-and why do we care? Chimerism. 2010;1(1):21–2.
Gupta AO, Wagner JE. Umbilical cord blood transplants: current status and evolving therapies. Front Pediatr. 2020;8:570282.
Scaradavou A, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121(5):752–8.
Chen J, et al. Long-term adaptation to hypoxia preserves hematopoietic stem cell function. Exp Hematol. 2016;44(9):866-873.e4.
Zimran E, Papa L, Hoffman R. Ex vivo expansion of hematopoietic stem cells: Finally transitioning from the lab to the clinic. Blood Rev. 2021:100853.
Gartner S, Kaplan HS. Long-term culture of human bone marrow cells. Proc Natl Acad Sci U S A. 1980;77(8):4756–9.
Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966;44(3):287–99.
Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82(7):2031–7.
Ivanovic Z, et al. Simultaneous maintenance of human cord blood SCID-repopulating cells and expansion of committed progenitors at low O2 concentration (3%). Stem Cells. 2004;22(5):716–24.
Shima H, et al. Reconstitution activity of hypoxic cultured human cord blood CD34-positive cells in NOG mice. Biochem Biophys Res Commun. 2009;378(3):467–72.
Kobayashi H, et al. Environmental optimization enables maintenance of quiescent hematopoietic stem cells ex vivo. Cell Rep. 2019;28(1):145158.e9. This article demonstrates HSCs can be kept quiescent in culture for 1 month, a promising route for ex vivo stem cell expansion.
Kobayashi, H, Takubo K. Protocol for the maintenance of quiescent murine hematopoietic stem cells. STAR Protoc. 2020;1(2):100078.
Kobayashi H, Takubo K. A culture method to maintain quiescent human hematopoietic stem cells. J Vis Exp. 2021;(171).
Danet GH, et al. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112(1):126–35.
Hammoud M, et al. Combination of low O(2) concentration and mesenchymal stromal cells during culture of cord blood CD34(+) cells improves the maintenance and proliferative capacity of hematopoietic stem cells. J Cell Physiol. 2012;227(6):2750–8.
Forristal CE, et al. HIF-1α is required for hematopoietic stem cell mobilization and 4-prolyl hydroxylase inhibitors enhance mobilization by stabilizing HIF-1α. Leukemia. 2015;29(6):1366–78.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Paige Dausinas Ni, Chris Basile, Chase Junge, Melissa Hartman, and Heather O’Leary declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cell:Cell Interactions in Stem Cell Maintenance
Rights and permissions
About this article
Cite this article
Dausinas Ni, P., Basile, C., Junge, C. et al. Hypoxia and Hematopoiesis. Curr Stem Cell Rep 8, 24–34 (2022). https://doi.org/10.1007/s40778-021-00203-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-021-00203-8