Abstract
Purpose of Review
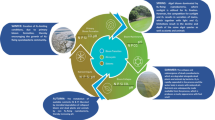
Cyanobacteria, commonly known as blue-green algae, are often seen as a problem. Their accumulation (bloom) in surface water can cause toxicity and aesthetic concerns. Efforts have been made in preventing and managing cyanobacterial blooms. By contrast, purposeful cultivation of cyanobacteria can create great opportunities in food, chemical and biofuel applications. This review summarises the current stage of research and the socio-economic impacts associated with both the problems and opportunities induced from the presence of cyanobacteria in surface water.
Recent Findings
Insightful knowledge of factors that trigger cyanobacterial blooms has allowed for the development of prevention and control strategies. Advanced technologies are utilised to detect, quantify and treat cyanobacterial biomass and cyanotoxins in a timely manner. Additionally, understanding of cyanobacterial biochemical properties enables their applications in food and health industry, agriculture and biofuel production. Researchers have been able to genetically modify several cyanobacterial strains to obtain a direct pathway for ethanol and hydrogen production.
Summary
Cyanobacterial blooms have been effectively addressed with advances technologies and cyanobacterial research. However, this review identified a knowledge gap regarding cyanotoxin synthesis and the relevant environmental triggers. This information is essential for developing measures to prevent cyanobacterial blooms. Additionally, this review affirms the promising opportunities that cyanobacteria offer in the food, cosmetics, pigments and agriculture. Biofuel production from cyanobacterial biomass presents an immense potential but is currently constrained by the cultivation process. Thus, future research should strive to achieve effective mass harvesting of cyanobacterial biomass and obtain a profound understanding of cyanotoxin production.





Similar content being viewed by others
References
Whitton BA, Potts M. Introduction to the cyanobacteria. In: Whitton BA, editor. Ecology of cyanobacteria II: their diversity in space and time. Dordrecht: Springer Netherlands; 2012. p. 1–13.
Huisman J, Codd GA, Paerl HW, Ibelings BW, Verspagen JMH, Visser PM. Cyanobacterial blooms. Nat Rev Microbiol. 2018;16(8):471–83. https://doi.org/10.1038/s41579-018-0040-1.
Introduction to the cyanobacteria University of California Museum of Paleontology. https://ucmp.berkeley.edu/bacteria/cyanointro.html. Accessed 11/11 2019.
NHMRC. Guidelines for managing risk in recreational waters Canberra: National Health and Medical Research Council; 2008.
Qin B, Li W, Zhu G, Zhang Y, Wu T, Gao G. Cyanobacterial bloom management through integrated monitoring and forecasting in large shallow eutrophic Lake Taihu (China). J Hazard Mater. 2015;287:356–63. https://doi.org/10.1016/j.jhazmat.2015.01.047.
Teixeira M, Costa M, Carvalho V, Pereira M, Hage E. Gastroenteritis epidemic in the area of the Itaparica Dam, Bahia, Brazil. Bull Pan Am Health Organ. 1993;27:244–53.
Steffensen DA. Economic cost of cyanobacterial blooms. In: Hudnell HK, editor. Cyanobacterial harmful algal blooms: state of the science and research needs. New York: Springer New York; 2008. p. 855–65.
CSIRO. Blue-green algae research 2019. https://www.csiro.au/en/Research/Environment/Water/Blue-green-algae/Our-research.
Zervou S-K, Christophoridis C, Kaloudis T, Triantis TM, Hiskia A. New SPE-LC-MS/MS method for simultaneous determination of multi-class cyanobacterial and algal toxins. J Hazard Mater. 2017;323:56–66. https://doi.org/10.1016/j.jhazmat.2016.07.020.
Sendall B, Reardon K, Hunt L, Menjivar T, Muhid P. Identification, detection & characterisation of cyanobacteria using traditional & DNA-based methods: forensic and scientific services, Queensland Government. 2019.
Udayan A, Arumugam M, Pandey A. Chapter 4 - nutraceuticals from algae and cyanobacteria. In: Rastogi RP, Madamwar D, Pandey A, editors. Algal green chemistry. Amsterdam: Elsevier; 2017. p. 65–89.
Morone J, Alfeus A, Vasconcelos V, Martins R. Revealing the potential of cyanobacteria in cosmetics and cosmeceuticals — a new bioactive approach. Algal Res. 2019;41:101541. https://doi.org/10.1016/j.algal.2019.101541.
Pathak J, Rajneesh MPK, Singh SP, Häder D-P, Sinha RP. Cyanobacterial farming for environment friendly sustainable agriculture practices: innovations and perspectives. Front Environ Sci. 2018;6(7). https://doi.org/10.3389/fenvs.2018.00007.
Gao Z, Zhao H, Li Z, Tan X, Lu X. Photosynthetic production of ethanol from carbon dioxide in genetically engineered cyanobacteria. Energy Environ Sci. 2012;5(12):9857–65. https://doi.org/10.1039/C2EE22675H.
Atsumi S, Hanai T, Liao JC. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature. 2008;451(7174):86–9. https://doi.org/10.1038/nature06450.
Allahverdiyeva Y, Leino H, Saari L, Fewer DP, Shunmugam S, Sivonen K, et al. Screening for biohydrogen production by cyanobacteria isolated from the Baltic Sea and Finnish lakes. Int J Hydrog Energy. 2010;35(3):1117–27. https://doi.org/10.1016/j.ijhydene.2009.12.030.
Mowe MAD, Mitrovic SM, Lim RP, Furey A, Yeo DCJ. Tropical cyanobacterial blooms: a review of prevalence, problem taxa, toxins and influencing environmental factors. J Limnol. 2015;74:205–24. https://doi.org/10.4081/jlimnol.2014.1005.
Visser PM, Ibelings BW, Bormans M, Huisman JJAE. Artificial mixing to control cyanobacterial blooms: a review. Aquat Ecol. 2016;50(3):423–41. https://doi.org/10.1007/s10452-015-9537-0.
Chakdar H, Jadhav S, Dhar D, Pabbi S. Potential applications of blue green algae. J Sci Ind Res. 2012;71:13–20. http://hdl.handle.net/123456789/13322.
Markou G, Georgakakis D. Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewaters: a review. Appl Energy. 2011;88(10):3389–401. https://doi.org/10.1016/j.apenergy.2010.12.042.
Vincent WF. Cyanobacteria. In: Likens GE, editor. Encyclopedia of inland waters. Oxford: Academic Press; 2009. p. 226–32.
Mur LR, Skulberg OM, Utkilen H. Cyanobacteria in the environment. In: Chorus I, Bartram J, editors. Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management: World Health Organization; 1999.
Cyanobacteria. Landcare Research https://www.landcareresearch.co.nz/resources/identification/algae/identification-guide/identify/guide/descriptions/cyanobacteria.
Manisha M. Cyanobacteria: occurrence, morphology and cell structure. http://www.biologydiscussion.com/bacteria/cyanobacteria/cyanobacteria-occurrence-morphology-and-cell-structure/52036.
Reynolds CS, Oliver RL, Walsby AE. Cyanobacterial dominance: the role of buoyancy regulation in dynamic lake environments. New Zeal J Mar Fresh. 1987;21(3):379–90. https://doi.org/10.1080/00288330.1987.9516234.
Kumar K, Dasgupta CN, Nayak B, Lindblad P, Das D. Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresour Technol. 2011;102(8):4945–53. https://doi.org/10.1016/j.biortech.2011.01.054.
Upendar G, Singh S, Chakrabarty J, Chandra Ghanta K, Dutta S, Dutta A. Sequestration of carbon dioxide and production of biomolecules using cyanobacteria. J Environ Manag. 2018;218:234–44. https://doi.org/10.1016/j.jenvman.2018.04.031.
Atia A, Saad A. Review on freshwater blue-green algae (cyanobacteria): occurrence, classification and toxicology. Biosci Biotechnol Res Asia. 2014;11:1319–25. https://doi.org/10.13005/bbra/1522.
Smith VH. Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton. Science. 1983;221(4611):669–71. https://doi.org/10.1126/science.221.4611.669.
Dao TS, Nimptsch J, Wiegand C. Dynamics of cyanobacteria and cyanobacterial toxins and their correlation with environmental parameters in Tri An Reservoir, Vietnam. J Water Health. 2016;14(4):699–712. https://doi.org/10.2166/wh.2016.257.
O’Neil JM, Davis TW, Burford MA, Gobler CJ. The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae. 2012;14:313–34. https://doi.org/10.1016/j.hal.2011.10.027.
Paerl HW, Huisman J. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ Microbiol Rep. 2009;1(1):27–37. https://doi.org/10.1111/j.1758-2229.2008.00004.x.
Watson SB, McCauley E, Downing JA. Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnol Oceanogr. 1997;42(3):487–95. https://doi.org/10.4319/lo.1997.42.3.0487.
Trimbee AM, Prepas EE. Evaluation of total phosphorus as a predictor of the relative biomass of blue-green algae with emphasis on Alberta lakes. Can J Fish Aquat Sci. 1987;44(7):1337–42. https://doi.org/10.1139/f87-158.
Merel S, Walker D, Chicana R, Snyder S, Baurès E, Thomas O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ Int. 2013;59:303–27. https://doi.org/10.1016/j.envint.2013.06.013.
Montechiaro F, Giordano M. Effect of prolonged dark incubation on pigments and photosynthesis of the cave-dwelling cyanobacterium Phormidium autumnale (Oscillatoriales, Cyanobacteria). Phycologia. 2006;45(6):704–10. https://doi.org/10.2216/06-15.1.
Visser PM, Verspagen JMH, Sandrini G, Stal LJ, Matthijs HCP, Davis TW, et al. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae. 2016;54:145–59. https://doi.org/10.1016/j.hal.2015.12.006.
Paerl HW. Mitigating harmful cyanobacterial blooms in a human- and climatically-impacted world. Life (Basel). 2014;4(4):988–1012. https://doi.org/10.3390/life4040988.
Wu T, Qin B, Brookes JD, Shi K, Zhu G, Zhu M, et al. The influence of changes in wind patterns on the areal extension of surface cyanobacterial blooms in a large shallow lake in China. Sci Total Environ. 2015;518–519:24–30. https://doi.org/10.1016/j.scitotenv.2015.02.090.
EPA, editor. Cyanobacteria and cyanotoxins: information for drinking water systems. Washington, DC; 2014.
Sanseverino I, Conduto António DS, Loos R, Lettieri T. Cyanotoxins: methods and approaches for their analysis and detection. Publications Office of the European Union. 2017. doi:http://doi.org/10.2760/630736 (print) http://doi.org/10.2760/36186 (online).
Zanchett G, Oliveira-Filho EC. Cyanobacteria and cyanotoxins: from impacts on aquatic ecosystems and human health to anticarcinogenic effects. Toxins (Basel). 2013;5(10):1896–917. https://doi.org/10.3390/toxins5101896.
D’Anglada LV, Strong J. Drinking water health advisory for the cyanobacterial toxin cylindrospermopsin. In: EPA US, editor. Washington, DC 2015.
Rosen BH. Understanding cyanobacterial ecological strategies p. https://www.epa.gov/sites/production/files/2016-05/documents/webinar-understanding-cyanobacterial.pdf.
Negri AP, Jones GJ. Bioaccumulation of paralytic shellfish poisoning (PSP) toxins from the cyanobacterium Anabaena circinalis by the freshwater mussel Alathyria condola. Toxicon. 1995;33(5):667–78. https://doi.org/10.1016/0041-0101(94)00180-G.
Wiese M, D'Agostino PM, Mihali TK, Moffitt MC, Neilan BA. Neurotoxic alkaloids: saxitoxin and its analogs. Mar Drugs. 2010;8(7):2185–211. https://doi.org/10.3390/md8072185.
Otten TG, Paerl HW. Health effects of toxic cyanobacteria in U.S. drinking and recreational waters: our current understanding and proposed direction. Curr Environ Health Rep. 2015;2(1):75–84. https://doi.org/10.1007/s40572-014-0041-9.
Sivonen K, Jones G. Cyanobacterial toxins. In: Bartram J, editor. Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management: World Health Organization; 1999.
Rzymski P, Poniedziałek B. Review paper: dermatotoxins synthesized by blue-green algae (cyanobacteria). Adv Dermatol Allergol. 2012;29(1):47–50.
Fujiki H, Suganuma M, Suguri H, Yoshizawa S, Takagi K, Nakayasu M et al. New tumor promoters from marine natural products. Marine toxins. ACS Symposium Series, vol 418: American Chemical Society; 1990. p. 232–40.
Chen Y, Yu S, Yang JB. Microcystins in drinking water and cancer mortality in a city along Taihu Lake. China Oncol. 2002;12:485–8.
Guidelines for drinking-water quality: forth edition incorporating the first addendum. 4th ed: World Health Organization; 2017.
D’Anglada LV, Donohue JM, Strong J, Hawkins B. In: EPA US, editor. Health effects support document for the cyanobacterial toxin microcystins. Washington, DC; 2015.
NHMRC, NHMMC. Australian drinking water guidelines paper 6 national water quality management strategy. Canberra: National Health and Medical Research Council, National Resource Management Ministerial Council; 2011.
Mazur-Marzec H, Sutryk K, Kobos J, Hebel A, Hohlfeld N, Błaszczyk A, et al. Occurrence of cyanobacteria and cyanotoxin in the Southern Baltic Proper. Filamentous cyanobacteria versus single-celled picocyanobacteria. Hydrobiologia. 2013;701(1):235–52. https://doi.org/10.1007/s10750-012-1278-7.
Volume 1: coastal and fresh waters. In: Guidelines for safe recreational water environments: World Health Organization; 2003.
Rücker J, Stüken A, Nixdorf B, Fastner J, Chorus I, Wiedner C. Concentrations of particulate and dissolved cylindrospermopsin in 21 Aphanizomenon-dominated temperate lakes. Toxicon. 2007;50(6):800–9. https://doi.org/10.1016/j.toxicon.2007.06.019.
Saker ML, Eaglesham GK. The accumulation of cylindrospermopsin from the cyanobacterium Cylindrospermopsis raciborskii in tissues of the Redclaw crayfish Cherax quadricarinatus. Toxicon. 1999;37(7):1065–77. https://doi.org/10.1016/S0041-0101(98)00240-2.
Humpage AR, Falconer IR. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male Swiss albino mice: determination of no observed adverse effect level for deriving a drinking water guideline value. Environ Toxicol. 2003;18(2):94–103. https://doi.org/10.1002/tox.10104.
Trainer VL, Hardy FJ. Integrative monitoring of marine and freshwater harmful algae in Washington State for public health protection. Toxins (Basel). 2015;7(4):1206–34. https://doi.org/10.3390/toxins7041206.
Salmaso N, Cerasino L, Boscaini A, Capelli C. Planktic Tychonema (Cyanobacteria) in the large lakes south of the Alps: phylogenetic assessment and toxigenic potential. FEMS Microbiol Ecol. 2016;92(10). https://doi.org/10.1093/femsec/fiw155.
Ibelings BW, Backer LC, Kardinaal WEA, Chorus I. Current approaches to cyanotoxin risk assessment and risk management around the globe. Harmful Algae. 2015;49:63–74. https://doi.org/10.1016/j.hal.2014.10.002.
Solter PF, Beasley VR. Chapter 38 - phycotoxins. In: Haschek WM, Rousseaux CG, Wallig MA, editors. Haschek and Rousseaux’s handbook of toxicologic pathology. 3rd ed. Boston: Academic Press; 2013. p. 1155–86.
Lajeunesse A, Segura PA, Gélinas M, Hudon C, Thomas K, Quilliam MA, et al. Detection and confirmation of saxitoxin analogues in freshwater benthic Lyngbya wollei algae collected in the St. Lawrence River (Canada) by liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2012;1219:93–103. https://doi.org/10.1016/j.chroma.2011.10.092.
Jüttner F, Watson SB. Biochemical and ecological control of Geosmin and 2-Methylisoborneol in source waters. Appl Environ Microbiol. 2007;73(14):4395–406. https://doi.org/10.1128/AEM.02250-06.
Izaguirre G, Hwang CJ, Krasner SW, McGuire MJ. Geosmin and 2-Methylisoborneol from cyanobacteria in three water supply systems. Appl Environ Microbiol. 1982;43(3):708–14.
Young WF, Horth H, Crane R, Ogden T, Arnott M. Taste and odour threshold concentrations of potential potable water contaminants. Water Res. 1996;30(2):331–40. https://doi.org/10.1016/0043-1354(95)00173-5.
Richardson K. Harmful or exceptional phytoplankton blooms in the marine ecosystem. In: Blaxter JHS, Southward AJ, editors. Advances in marine biology: Academic Press; 1997. p. 301–85.
Kong P, Moorman GW, Lea-Cox JD, Ross DS, Richardson PA, Hong C. Zoosporic tolerance to pH stress and its implications for Phytophthora species in aquatic ecosystems. Appl Environ Microbiol. 2009;75(13):4307–14. https://doi.org/10.1128/AEM.00119-09.
Atli G, Canli EG, Eroglu A, Canli M. Characterization of antioxidant system parameters in four freshwater fish species. Ecotoxicol Environ Saf. 2016;126:30–7. https://doi.org/10.1016/j.ecoenv.2015.12.012.
Schmetterer G. Cyanobacterial respiration. In: Bryant DA, editor. The molecular biology of cyanobacteria. Dordrecht: Springer Netherlands; 1994. p. 409–35.
Sanseverino I, Conduto António DS, Pozzoli L, Dobricic S, Lettieri T. Algal bloom and its economic impact. 2016.
Lopez CB, Jewett EB, Dortch Q, Walton BT, Hudnell HK. Scientific assessment of freshwater harmful algal bloom. Washington, DC; 2008.
Pretty JN, Mason CF, Nedwell DB, Hine RE, Leaf S, Dils R. Environmental costs of freshwater eutrophication in England and Wales. Environ Sci Technol. 2003;37(2):201–8. https://doi.org/10.1021/es020793k.
Dodds WK, Bouska WW, Eitzmann JL, Pilger TJ, Pitts KL, Riley AJ, et al. Eutrophication of U.S. freshwaters: analysis of potential economic damages. Environ Sci Technol. 2009;43(1):12–9. https://doi.org/10.1021/es801217q.
Kouzminov A, Ruck J, Wood SA. New Zealand risk management approach for toxic cyanobacteria in drinking water. 2007;31(3):275–81. https://doi.org/10.1111/j.1467-842X.2007.00061.x.
Ibelings BW, Fastner J, Bormans M, Visser PMJAE. Cyanobacterial blooms. Ecology, prevention, mitigation and control: editorial to a CYANOCOST Special Issue. Aquat Ecol. 2016;50(3):327–31. https://doi.org/10.1007/s10452-016-9595-y.
Schindler DW. Eutrophication and recovery in experimental lakes: implications for lake management. Science. 1974;184(4139):897–9. https://doi.org/10.1126/science.184.4139.897.
Jeppesen E, Jensen JP, Søndergaard M. Response of phytoplankton, zooplankton, and fish to re-oligotrophication: an 11 year study of 23 Danish lakes. Aquat Ecosyst Health. 2002;5(1):31–43. https://doi.org/10.1080/14634980260199945.
Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ, et al. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci. 2008;105(32):11254–8. https://doi.org/10.1073/pnas.0805108105.
Paerl HW, Hall NS, Calandrino ES. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci Total Environ. 2011;409(10):1739–45. https://doi.org/10.1016/j.scitotenv.2011.02.001.
Harke MJ, Gobler CJ. Global transcriptional responses of the toxic cyanobacterium, Microcystis aeruginosa, to nitrogen stress, phosphorus stress, and growth on organic matter. PLoS One. 2013;8(7):e69834. https://doi.org/10.1371/journal.pone.0069834.
Müller S, Mitrovic SM. Phytoplankton co-limitation by nitrogen and phosphorus in a shallow reservoir: progressing from the phosphorus limitation paradigm. Hydrobiologia. 2015;744(1):255–69. https://doi.org/10.1007/s10750-014-2082-3.
Moore BC, Cross BK, Beutel M, Dent S, Preece E, Swanson M. Newman Lake restoration: a case study part III. Hypolimnetic oxygenation. Lake Reservoir Manage. 2012;28(4):311–27. https://doi.org/10.1080/07438141.2012.738463.
Toffolon M, Ragazzi M, Righetti M, Teodoru CR, Tubino M, Defrancesco C, et al. Effects of artificial hypolimnetic oxygenation in a shallow lake. Part 1: phenomenological description and management. J Environ Manag. 2013;114:520–9. https://doi.org/10.1016/j.jenvman.2012.10.062.
Bormans M, Maršálek B, Jančula DJAE. Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: a review. 2016;50(3):407–22. https://doi.org/10.1007/s10452-015-9564-x.
Douglas GB, Adeney JA, Robb M. A novel technique for reducing bioavailable phosphorus in water and sediments. International Association Water Quality Conference on Diffuse Pollution1999. p. 517–523.
Robb M, Greenop B, Goss Z, Douglas G, Adeney J. Application of PhoslockTM, an innovative phosphorus binding clay, to two Western Australian waterways: preliminary findings. Hydrobiologia. 2003;494(1):237–43. https://doi.org/10.1023/a:1025478618611.
Copetti D, Finsterle K, Marziali L, Stefani F, Tartari G, Douglas G, et al. Eutrophication management in surface waters using lanthanum modified bentonite: a review. Water Res. 2016;97:162–74. https://doi.org/10.1016/j.watres.2015.11.056.
Stroom JM, Kardinaal WEA. How to combat cyanobacterial blooms: strategy toward preventive lake restoration and reactive control measures. Aquat Ecol. 2016;50(3):541–76. https://doi.org/10.1007/s10452-016-9593-0.
Bullerjahn GS, McKay RM, Davis TW, Baker DB, Boyer GL, D’Anglada LV, et al. Global solutions to regional problems: collecting global expertise to address the problem of harmful cyanobacterial blooms. A Lake Erie case study. Harmful Algae. 2016;54:223–38. https://doi.org/10.1016/j.hal.2016.01.003.
Tátrai I, Mátyás K, Korponai J, Paulovits G, Pomogyi P. The role of the Kis-Balaton water protection system in the control of water quality of Lake Balaton. Ecol Eng. 2000;16(1):73–8. https://doi.org/10.1016/S0925-8574(00)00091-4.
Park J, Church J, Son Y, Kim K-T, Lee WH. Recent advances in ultrasonic treatment: challenges and field applications for controlling harmful algal blooms (HABs). Ultrason Sonochem. 2017;38:326–34. https://doi.org/10.1016/j.ultsonch.2017.03.003.
Rajasekhar P, Fan L, Nguyen T, Roddick FA. A review of the use of sonication to control cyanobacterial blooms. Water Res. 2012;46(14):4319–29. https://doi.org/10.1016/j.watres.2012.05.054.
Suslick KS. Sonochemistry. Science. 1990;247(4949):1439–45. https://doi.org/10.1126/science.247.4949.1439.
Schneider OD, Weinrich LA, Brezinski S. Ultrasonic treatment of algae in a New Jersey reservoir. J Am Water Works Assoc. 2015;107(10):E533–E42. https://doi.org/10.5942/jawwa.2015.107.0149.
Mishra S, Stumpf RP, Schaeffer BA, Werdell PJ, Loftin KA, Meredith A. Measurement of cyanobacterial bloom magnitude using satellite remote sensing. Sci Rep. 2019;9(1):18310. https://doi.org/10.1038/s41598-019-54453-y.
Bui MH, Pham TL, Dao TS. Prediction of cyanobacterial blooms in the Dau Tieng Reservoir using an artificial neural network. Mar Freshw Res. 2017;68(11):2070–80. https://doi.org/10.1071/MF16327.
Schaeffer B, Loftin K, Stumpf R, Werdell J. Agencies collaborate, develop a cyanobacteria assessment network. Eos. 2015;96. https://doi.org/10.1029/2015EO038809.
Algae. WaterNSW. 2019. https://www.waternsw.com.au/water-quality/algae#stay.
Treatment options. In: Newcombe G, editor. International guidance manual for the management of toxic cyanobacteria. United Kingdom: Global Water Research Coalition; 2009.
Waters T, Dugan A, Lieberman R, Speth T, Carroll G. In: EPA, editor. Water treatment optimization for cyanotoxins. Washington, DC: In; 2016.
Singh G, Patidar SK. Microalgae harvesting techniques: a review. J Environ Manag. 2018;217:499–508. https://doi.org/10.1016/j.jenvman.2018.04.010.
Griffiths MJ, Dicks RG, Richardson C, Harrison ST. Advantages and challenges of microalgae as a source of oil for biodiesel. In: Stoytcheva M, Montero G, editors. Biodiesel - feedstocks and processing technologies. InTechOpen; 2011.
Barros AI, Gonçalves AL, Simões M, Pires JCM. Harvesting techniques applied to microalgae: a review. Renew Sust Energ Rev. 2015;41:1489–500. https://doi.org/10.1016/j.rser.2014.09.037.
Fasaei F, Bitter JH, Slegers PM, van Boxtel AJB. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018;31:347–62. https://doi.org/10.1016/j.algal.2017.11.038.
Zaffiro A, Rosenblum L, Wendelken SC. Method 546: determination of total microcystins and nodularins in drinking water and ambient water by enzyme-linked immunosorbent assay: U.S. EPA; 2015.
Gaget V, Lau M, Sendall B, Froscio S, Humpage AR. Cyanotoxins: which detection technique for an optimum risk assessment? Water Res. 2017;118:227–38. https://doi.org/10.1016/j.watres.2017.04.025.
Rudi K, Skulberg OM, Skulberg R, Jakobsen KS. Application of sequence-specific labeled 16S rRNA gene oligonucleotide probes for genetic profiling of cyanobacterial abundance and diversity by array hybridization. Appl Environ Microbiol. 2000;66(9):4004–11. https://doi.org/10.1128/aem.66.9.4004-4011.2000.
Rantala A, Rizzi E, Castiglioni B, De Bellis G, Sivonen K. Identification of hepatotoxin-producing cyanobacteria by DNA-chip. Environ Microbiol. 2008;10(3):653–64. https://doi.org/10.1111/j.1462-2920.2007.01488.x.
EPA. Detection methods for cyanotoxins. 2005. https://www.epa.gov/ground-water-and-drinking-water/detection-methods-cyanotoxins.
Moreira C, Ramos V, Azevedo J, Vasconcelos V. Methods to detect cyanobacteria and their toxins in the environment. Appl Microbiol Biotechnol. 2014;98(19):8073–82. https://doi.org/10.1007/s00253-014-5951-9.
Kamp L, Church JL, Carpino J, Faltin-Mara E, Rubio F. The effects of water sample treatment, preparation, and storage prior to cyanotoxin analysis for cylindrospermopsin, microcystin and saxitoxin. Chem Biol Interact. 2016;246:45–51. https://doi.org/10.1016/j.cbi.2015.12.016.
Gurbuz F, Metcalf JS, Codd GA, Karahan AG. Evaluation of enzyme-linked immunosorbent assays (ELISAs) for the determination of microcystins in cyanobacteria. Environ Forensic. 2012;13(2):105–9. https://doi.org/10.1080/15275922.2012.676596.
Aguilar M-I. Reversed-phase high-performance liquid chromatography. In: Aguilar M-I, editor. HPLC of peptides and proteins: methods and protocols. Totowa: Springer New York; 2004. p. 9–22.
Dell’Aversano C, Hess P, Quilliam MA. Hydrophilic interaction liquid chromatography–mass spectrometry for the analysis of paralytic shellfish poisoning (PSP) toxins. J Chromatogr A. 2005;1081(2):190–201. https://doi.org/10.1016/j.chroma.2005.05.056.
Chen J, Gao L, Li Z, Wang S, Li J, Cao W, et al. Simultaneous screening for lipophilic and hydrophilic toxins in marine harmful algae using a serially coupled reversed-phase and hydrophilic interaction liquid chromatography separation system with high-resolution mass spectrometry. Anal Chim Acta. 2016;914:117–26. https://doi.org/10.1016/j.aca.2016.01.062.
Newcombe G, Nicholson B. Treatment options for the saxitoxin class of cyanotoxins. Water Supply. 2002;2(5–6):271–5. https://doi.org/10.2166/ws.2002.0179.
Nimptsch J, Wiegand C, Pflugmacher S. Cyanobacterial toxin elimination via bioaccumulation of MC-LR in aquatic macrophytes: an application of the “Green Liver Concept”. Environ Sci Technol. 2008;42(22):8552–7. https://doi.org/10.1021/es8010404.
Pflugmacher S, Kühn S, Lee S, Choi J, Baik S, Kwon K, et al. Green Liver Systems® for water purification: using the phytoremediation potential of aquatic macrophytes for the removal of different cyanobacterial toxins from water. Am J Plant Sci. 2015;6:1607–18. https://doi.org/10.4236/ajps.2015.69161.
Trung B, Dao TS, Faassen E, Lürling M. Cyanobacterial blooms and microcystins in Southern Vietnam. Toxins (Basel). 2018;10(11):471. https://doi.org/10.3390/2Ftoxins10110471.
Dillon JC, Phuc AP, Dubacq JP. Nutritional value of the alga Spirulina. In: Simopoulos AP, editor. Plants in human nutrition. Basel: Karger; 1995. p. 32–46.
Liang S, Liu X, Chen F, Chen Z. Current microalgal health food R & D activities in China. In: Ang PO, editor. Asian Pacific phycology in the 21st century: prospects and challenges. Developments in hydrobiology. Dordrecht: Springer Netherlands; 2004. p. 45–8.
Yamaguchi K. Recent advances in microalgal bioscience in Japan, with special reference to utilization of biomass and metabolites: a review. J Appl Phycol. 1996;8(6):487–502. https://doi.org/10.1007/bf02186327.
Spolaore P, Joannis-Cassan C, Duran E, Isambert A. Commercial applications of microalgae. J Biosci Bioeng. 2006;101(2):87–96. https://doi.org/10.1263/jbb.101.87.
Milledge JJ. Commercial application of microalgae other than as biofuels: a brief review. Rev Environ Sci Biotechnol. 2011;10(1):31–41. https://doi.org/10.1007/s11157-010-9214-7.
Oren A, Gunde-Cimerman N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol Lett. 2007;269(1):1–10. https://doi.org/10.1111/j.1574-6968.2007.00650.x.
Kageyama H, Waditee-Sirisattha R. Chapter 5 - mycosporine-like amino acids as multifunctional secondary metabolites in cyanobacteria: from biochemical to application aspects. In: Atta ur R, editor. Studies in natural products chemistry: Elsevier; 2018. p. 153–94.
Singh R, Parihar P, Singh M, Bajguz A, Kumar J, Singh S, et al. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: current status and future prospects. Front Microbiol. 2017;8(515). https://doi.org/10.3389/fmicb.2017.00515.
Liu Z, Häder DP, Sommaruga R. Occurrence of mycosporine-like amino acids (MAAs) in the bloom-forming cyanobacterium Microcystis aeruginosa. J Plankton Res. 2004;26(8):963–6. https://doi.org/10.1093/plankt/fbh083.
Bhatia S, Garg A, Sharma K, Kumar S, Sharma A, Purohit AP. Mycosporine and mycosporine-like amino acids: a paramount tool against ultra violet irradiation. Pharmacogn Rev. 2011;5(10):138–46. https://doi.org/10.4103/0973-7847.91107.
Stevenson CS, Capper EA, Roshak AK, Marquez B, Grace K, Gerwick WH, et al. Scytonemin-a marine natural product inhibitor of kinases key in hyperproliferative inflammatory diseases. Inflamm Res. 2002;51(2):112–4. https://doi.org/10.1007/bf02684014.
Rastogi RP, Sonani RR, Madamwar D. Cyanobacterial sunscreen Scytonemin: role in photoprotection and biomedical research. Appl Biochem Biotechnol. 2015;176(6):1551–63. https://doi.org/10.1007/s12010-015-1676-1.
De Philippis R, Sili C, Paperi R, Vincenzini M. Exopolysaccharide-producing cyanobacteria and their possible exploitation: a review. J Appl Phycol. 2001;13(4):293–9. https://doi.org/10.1023/A:1017590425924.
Okajima MK, Bamba T, Kaneso Y, Hirata K, Fukusaki E, Kajiyama S, et al. Supergiant ampholytic sugar chains with imbalanced charge ratio form saline ultra-absorbent hydrogels. Macromolecules. 2008;41(12):4061–4. https://doi.org/10.1021/ma800307w.
Okajima MK, Miyazato S, Kaneko T. Cyanobacterial megamolecule sacran efficiently forms LC gels with very heavy metal ions. Langmuir. 2009;25(15):8526–31. https://doi.org/10.1021/la8036956.
Saini DK, Pabbi S, Shukla P. Cyanobacterial pigments: perspectives and biotechnological approaches. Food Chem Toxicol. 2018;120:616–24. https://doi.org/10.1016/j.fct.2018.08.002.
Pabbi S. Blue green algae: a potential biofertilizer for rice. In: Sahoo D, Seckbach J, editors. The algae world. Dordrecht: Springer Netherlands; 2015. p. 449–65.
Singh JS, Kumar A, Rai AN, Singh DP. Cyanobacteria: a precious bio-resource in agriculture, ecosystem, and environmental sustainability. 2016;7(529). https://doi.org/10.3389/fmicb.2016.00529.
Issa A, Abd-Alla M, Ohyama T. Nitrogen fixing cyanobacteria: future prospect. In: Ohyama T, editor. Advances in biology and ecology of nitrogen fixation. InTechOpen; 2014. p. 23–48.
Rai AK, Sharma NK. Phosphate metabolism in the cyanobacterium Anabaena doliolum under salt stress. Curr Microbiol. 2006;52(1):6–12. https://doi.org/10.1007/s00284-005-0043-9.
Ghazal FM, Mahdy E-SM, MSA EL-F, EL-Sadany AEGY, Doha NME. The use of cyanobacteria as biofertilizer in wheat cultivation under different nitrogen rates. Nat Sci. 2018;16(4):30–5. https://doi.org/10.7537/marsnsj160418.06.
Gebre E, Abdi T, Wolde-meskel E, Bulta A, Davis J. Response of kale (Brassica oleracea L) crop to cyanobacterial biofertilizer in Ziway area, Ethiopia. Journal of Biology, Agriculture and Healthcare. 2018;8(13).
Grzesik M, Romanowska-Duda Z, Kalaji HM. Effectiveness of cyanobacteria and green algae in enhancing the photosynthetic performance and growth of willow (Salix viminalis L.) plants under limited synthetic fertilizers application. Photosynthetica. 2017;55(3):510–21. https://doi.org/10.1007/s11099-017-0716-1.
Sarsekeyeva F, Zayadan BK, Usserbaeva A, Bedbenov VS, Sinetova MA, Los DA. Cyanofuels: biofuels from cyanobacteria. Reality and perspectives. Photosynth Res. 2015;125(1):329–40. https://doi.org/10.1007/s11120-015-0103-3.
Savakis P, Hellingwerf KJ. Engineering cyanobacteria for direct biofuel production from CO2. Curr Opin Biotechnol. 2015;33:8–14. https://doi.org/10.1016/j.copbio.2014.09.007.
Singh V, Chaudhary DK, Mani I, Dhar PK. Recent advances and challenges of the use of cyanobacteria towards the production of biofuels. Renew Sust Energ Rev. 2016;60:1–10. https://doi.org/10.1016/j.rser.2016.01.099.
Deng M-D, Coleman JR. Ethanol synthesis by genetic engineering in cyanobacteria. Appl Environ Microbiol. 1999;65(2):523–8.
Sveshnikov DA, Sveshnikova NV, Rao KK, Hall DO. Hydrogen metabolism of mutant forms of Anabaena variabilis in continuous cultures and under nutritional stress. FEMS Microbiol Lett. 1997;147(2):297–301. https://doi.org/10.1111/j.1574-6968.1997.tb10257.x.
Ali I, Basit MA. Significance of hydrogen content in fuel combustion. Int J Hydrog Energy. 1993;18(12):1009–11. https://doi.org/10.1016/0360-3199(93)90083-M.
Srirangan K, Pyne ME, Perry CC. Biochemical and genetic engineering strategies to enhance hydrogen production in photosynthetic algae and cyanobacteria. Bioresour Technol. 2011;102(18):8589–604. https://doi.org/10.1016/j.biortech.2011.03.087.
Cournac L, Guedeney G, Peltier G, Vignais PM. Sustained photoevolution of molecular hydrogen in a mutant of Synechocystis sp. strain PCC 6803 deficient in the type I NADPH-dehydrogenase complex. J Bacteriol. 2004;186(6):1737. https://doi.org/10.1128/JB.186.6.1737-1746.2003.
Yoshino F, Ikeda H, Masukawa H, Sakurai H. High photobiological hydrogen production activity of a Nostoc sp. PCC 7422 uptake hydrogenase-deficient mutant with high nitrogenase activity. Mar Biotechnol. 2007;9(1):101–12. https://doi.org/10.1007/s10126-006-6035-3.
Mona S, Kaushik A, Kaushik CP. Hydrogen production and metal-dye bioremoval by a Nostoc linckia strain isolated from textile mill oxidation pond. Bioresour Technol. 2011;102(3):3200–5. https://doi.org/10.1016/j.biortech.2010.11.005.
Boopathi T, Ki J-S. Impact of environmental factors on the regulation of cyanotoxin production. Toxins (Basel). 2014;6(7):1951–78. https://doi.org/10.3390/toxins6071951.
Alexova R, Haynes PA, Ferrari BC, Neilan BA. Comparative protein expression in different strains of the bloom-forming cyanobacterium Microcystis aeruginosa. Mol Cell Proteomics. 2011;10(9):M110.003749. https://doi.org/10.1074/mcp.M110.003749.
Camargo S, Valladares A, Flores E, Herrero A. Transcription activation by NtcA in the absence of consensus NtcA-binding sites in an anabaena heterocyst differentiation gene promoter. J Bacteriol. 2012;194(11):2939–48. https://doi.org/10.1128/JB.05994-11.
Burford MA, Davis TW, Orr PT, Sinha R, Willis A, Neilan BA. Nutrient-related changes in the toxicity of field blooms of the cyanobacterium. Cylindrospermopsis raciborskii. 2014;89(1):135–48. https://doi.org/10.1111/1574-6941.12341.
Tsuchiya S, Cho Y, Konoki K, Nagasawa K, Oshima Y, Yotsu-Yamashita M. Synthesis and identification of proposed biosynthetic intermediates of saxitoxin in the cyanobacterium Anabaena circinalis (TA04) and the dinoflagellate Alexandrium tamarense (Axat-2). Org Biomol Chem. 2014;12(19):3016–20. https://doi.org/10.1039/C4OB00071D.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Water Pollution
Rights and permissions
About this article
Cite this article
Vu, H.P., Nguyen, L.N., Zdarta, J. et al. Blue-Green Algae in Surface Water: Problems and Opportunities. Curr Pollution Rep 6, 105–122 (2020). https://doi.org/10.1007/s40726-020-00140-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-020-00140-w