Abstract
Seaweeds are a major contributor to global marine aquaculture production, with the biomass being mainly used, among others, for human nutrition, pharmaceutics, and cosmetics. However, green seaweeds are severely underrepresented, compared to red and brown macroalgae. Caulerpa lentillifera (known as “sea grapes” or “green caviar”) is an edible, green seaweed with a distinctive texture and various nutritional benefits. In this review, all articles on sea grapes published between 1900 and October 2022 and found in the scientific citation databases Scopus and Web of Science (search string: “caulerpa” AND “lentillifera”) were grouped by research topic and the intended application following the PRISMA approach. 51% of the 130 articles included in the review focused on the topic of “Biochemical composition”, followed by “Water treatment” (18%) and “Ecophysiology” (15%). The most prominent application was “Pharmaceutics”, followed by “Cultivation” and “Fundamental research”. In order to provide a knowledge base to researchers and practitioners of C. lentillifera aquaculture, research that was simultaneously grouped under one of the topics “Biochemical composition”, “Water treatment”, or “Ecophysiology” and the applications “Cultivation”, “Nutritional value” or “Post-harvest” was summarized in more detail. Light management of sea grapes, their use as a high-value co-culture species and the capacity to bioremediate nutrients, as well as their short shelf-life were identified as important areas of research interest. The assessment revealed several knowledge gaps, for example the need for intra-species comparisons of C. lentillifera biochemical composition across spatial and temporal scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Macroalgae represent > 50% of global marine and coastal aquaculture products (35 million t in 2020, based on wet weight (WW), mainly due to production for human consumption (FAO 2020; Chopin and Tacon 2021). Eight genera of red and brown macroalgae dominate the production, whereas green macroalgae are underrepresented with < 1% (FAO 2020; reviewed by Moreira et al. 2021). However, Caulerpa is one genus that is gaining increasing popularity, with the highest mean contribution to global green seaweed cultivation in the years 1950–2019 (annual average of 6404 t WW), but with declining values until 2019 (1090 t WW, Cai et al. 2021a). However, these production values are likely to be underestimated, mainly based on reports from The Philippines. Caulerpa species, and especially edible C. racemosa and C. lentillifera (known as “sea grapes” or “green caviar”) are particularly popular in the Indo-Pacific region, where they are consumed fresh or salt-preserved in salads or as a snack (Long et al. 2020b). In particular, the striking texture (Fig. 1A; Zubia et al. 2020), the nutritional benefits, including e.g. the content of bioactive compounds and polyunsaturated fatty acids (PUFA), and the pleasant taste have led to an increasing demand of sea grapes worldwide (de Gaillande et al. 2017; Chen et al. 2019; Zubia et al. 2020). Compared to average seaweeds, sea grapes achieve high market prices (Dobson et al. 2020; Cai et al. 2021a) and they are proposed as promising functional food ingredient (Syakilla et al. 2022), or certain compounds are being investigated in a pharmaceutical context, e.g. for their antidiabetic and anticancer activities (Daud et al. 2020; Fajriah et al. 2020; Manoppo et al. 2022). However, the species is not yet covered by the Novel food Regulation (EU), which limits the potential customer base (Barbier et al. 2019).
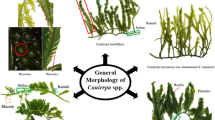
A Caulerpa lentillifera consists of upright fronds (assimilators) with grape-like, vesiculate ramuli irregularly arranged around a pedicel, which are attached to creeping stolons with rhizoids (Zubia et al., 2020). The life-cycle of the sea grapes in aquaculture consists of different stages E. Seedlings are applied to start the cultivation, which can take place in outdoor, tidal ponds B or in land-based systems. The shade-adapted seaweeds are shaded from the sun, e.g. with gauze material C. Sea grape fronds reaching harvestable size are continuously harvested during the cultivation season D and the harvest is collected at a collection point for cleaning and sorting of the product before retail of the fresh or dehydrated sea grapes G. C. lentillifera fronds are e.g. served with sushi or as a salad F. Pictures were taken at a sea grape farming facility in Vietnam, Khanh Hoa province
Historically, sea grape cultivation began in Japan (Okinawa) and The Philippines (Trono and Toma 1993; Yap 1999). In The Philippines sea grapes were introduced by accident into fish ponds, but the successful growth of the species ensured its targeted cultivation (Trono and Largo 2019).
As sea grapes can be propagated by fragmentation, they are easy to cultivate without the need for expensive infrastructure or strong expertise (Fig. 1B-F; de Gaillande et al. 2017). Cultivation methods vary according to country and system (Trono and Largo 2019). In The Philippines and Vietnam the algae are grown in perforated plastic trays or nets (tray method) or are planted directly into the sediment in tidal ponds (sowing method; Rabia 2016), sometimes shaded with e.g. gauze material (Fig. 1B,C). In Japan and China, land-based raceway cultures are increasingly used to meet the high demand for sea grapes (de Gaillande et al. 2017). However, sea grapes can also be grown in sheltered coastal areas in nets or trays (Tanduyan et al. 2013). The rapid growth and relatively low habitat requirements of sea grapes have also led to their increased use in integrated aquaculture systems (Paul and de Nys 2008), in order to mitigate the potentially problematic nutrient rich effluent of wastewaters and to provide an additional income from the metabolized biomass (Largo et al. 2016; Bambaranda et al. 2019a, b; Dobson et al. 2020). After harvesting of the edible fronds, they are soaked in tanks with seawater to allow this siphonous alga to heal tissue injuries. Subsequently, the fronds meeting the required quality standards (e.g. bright green colour, size) are stored in plastic containers with moisture sheets for shipment or retail as a fresh product, or for preservation (dehydrated or brine-cured de Gaillande et al. 2017; Terada et al. 2018; Chaiklahan et al. 2020). Biomass that does not meet food quality standards (60–70%) is discarded as waste, but there is potential for its further use (Chaiklahan et al. 2020).
Along with the economic interest, the number of scientific publications seems to be increasing. Recent review articles and book chapters focused on the consumption, nutritional value and farming of the genus Caulerpa (de Gaillande et al. 2017), as well as the biology and its use (Zubia et al. 2020) and the nutraceutical and pharmaceutical potential (Darmawan et al. 2020). To our knowledge one review article from 2019 sums up the research on the species C. lentillifera (Chen et al. 2019) and one review summarizes the nutrients, phytochemicals and health benefits (Syakilla et al. 2022). The present review article aims to (1) conduct a scientometric analysis of the published literature to identify trends of the different research topics and applications, in order to reveal knowledge gaps and identify future research directions. To achieve this goal, seven research topics (e.g. “Biochemical composition”, “Genetics”, “Water treatment”) and nine research applications (e.g. “Pharmaceutics”, “Fundamental research”, “Cultivation”) were formulated and the articles were grouped in the respective topic and application. In a next step (2), the literature focusing on the aquaculture of C. lentillifera, namely the topics of cultivation parameters, nutritional value, and post-harvest applications were summarized in concise manner to provide a structured overview for practitioners in the field and researchers working with this species.
Material and methods
Literature review
We conducted a systematic literature search using two popular scientific citation databases, namely Web of Science (WoS) and Scopus. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement was applied (Liberati et al. 2009). In both databases the search string “caulerpa” AND “lentillifera” for the period 1900 to 2022 was used to search within title, keywords and abstract. The search took place on 29 October 2022 and resulted in a total of n = 192 studies (after removal of duplicates, Fig. 2).
Flow diagram for the systematic literature review on Caulerpa lentillifera. The different stages for the identification, screening, and inclusion of relevant articles are shown. A template of the flow diagram was downloaded from https://www.prisma-statement.org
Selection criteria
In order to check for eligibility of the studies the following selection criteria were used a) Caulerpa lentillifera is a main topic of the article and b) language of the article was English, c) the article was not a review article, d) scientific accuracy was given. This was evaluated by screening the titles and abstracts of the documents. In case the information provided was not sufficient to determine the question, the complete document was screened.
Data extraction
From the studies declared “eligible” according to the criteria (n = 130, Fig. 2), the following information was extracted: (a) publication year, (b) study location, (c) affiliation of the main author, (d) location of the affiliation of the main author, (e) type of article (journal article, review article, book chapter, book, technical report), (f) topic of research (definitions in On-line Appendices I) and (g) application of research (definitions in On-line Appendices II). The topics and applications were defined by the authors after reviewing the existing literature. The search and extraction criteria were tested by a pilot classification, where two authors (BBC and LES) categorized 30 studies and discussed and cross-checked their choices in order to ensure a reliable and homogenized coding. Two papers, namely Stuthmann et al. (2020) and Paul et al. (2014) have been sorted in two categories since they dealt with various topics and/or applications (“Ecophysiology” – “Post-harvest” & “Ecophysiology” – “Cultivation” and “Biochemical composition” – “Nutritional value” & “Water treatment” – “Cultivation”, respectively) within each respective article.
This review was set-up on one hand as a scientometric analysis of the existing literature in order to identify research trends and knowledge gaps, and on the other hand as a contextual synopsis of sea grape aquaculture for practitioners and field-researchers. Hence, the review investigated certain combinations of topics and applications in more contextual detail, namely the topics of “Ecophysiology”, “Biochemical composition” and “Water treatment” with the respective applications of “Cultivation”, “Nutritional value” or “Post-harvest”. In a subsequent discussion, first the results of the scientometric analysis were considered and secondly the contextual summary was analysed across topics and applications with a focus on the peculiarities and knowledge gaps identified. The salinity units were reported as they appeared in the respective papers.
Results
Scientometric analysis: Number of publications, research topics and applications
Since 1990, eight review articles on the topic of C. lentillifera have been published, but all of them in the period 2019–2022, and six of them focused only on sea grapes. Until the search for this review (October 2022) 130 research articles were published (Fig. 3A). However, since 2018, the annual number of published journal articles about sea grapes was ≥ 10, and in total added up to 86 (66% of total published journal articles). The majority of articles were on the topics of “Biochemical composition” (51%), followed by “Water treatment”, “Ecophysiology” and “Genetics”, whereas only ≤ 5 articles researched “Microbiome”, “Distribution” and “Ethnophycology”, respectively (Fig. 3B). In contrast, the application of the research articles was more distributed, with “Pharmaceutics” having the highest and “Feedstock” and “Cosmetics” the lowest count, focusing on the use for bio-oil production (Ong et al. 2019; Wuttilerts et al. 2019) or in creams (Thu et al. 2018; Chang et al. 2021; Fig. 3C). The application as “Animal feed” was also underrepresented with only three studies, using sea grapes as fish (Ilias et al. 2015; Arisa et al. 2020) and shrimp (Putra et al. 2019) feed. The application of “Pharmaceutics” was made up almost exclusively by articles from the research topic “Biochemical composition”, majorly contributing to the high frequency of this topic (Fig. 4) and focusing on various bioactivities of the seaweed’s metabolites, including anti-inflammatory (Yoojam et al. 2021), anti-diabetic (Khairuddin et al. 2020) and anti-viral (You et al. 2022). “Industrial effluences” encompassed mainly articles, where C. lentillifera biomass was used as a bio-adsorbent for basic dyes (Marungrueng and Pavasant 2006, 2007; Pimol et al. 2008) and heavy metals (Apiratikul and Pavasant 2006, 2008; Pavasant et al. 2006; Apiratikul et al. 2011; Ahamad Zakeri and Abu Bakar 2013; Apiratikul 2017, 2020; Li et al. 2021). The application of “Fundamental research” encompassed all studies of the topics “Genetics” and “Distribution”, as well as a few on “Biochemical composition”, “Ecophysiology” and “Ethnophycology” (Fig. 4). Studies within the topic of “Genetics” focused e.g. on the alga’s chloroplast (Gao et al. 2018), mitochondrial (Zheng et al. 2018; Jia et al. 2019) and complete genome (Arimoto et al. 2019a) and DNA in their pyrenoid core (Miyamura and Hori 1991, 1995), as well as on population genetics (Benzie et al. 1997; Kazi et al. 2013). It accompanied research from the topic “Distribution”, reporting on (re)discoveries of C. lentillifera in the Gulf of Mannar (Mary et al. 2009) and Gulf of Kutch (Mantri 2004), India and Hainan Island, China (Gao et al. 2020). The topic “Microbiome” encompassed four studies, of which half were on the application of “Post-harvest”, namely the effect of season, washing (Pang et al. 2022) and petrifilm aerobic count plate (ACP, Kudaka et al. 2010) and the other half on “Cultivation”, dealing with the microbiome of healthy and diseased C. lentillifera (Liang et al. 2019; Kopprio et al. 2021).
Sankey-plot visualizing the distribution of Caulerpa lentillifera related articles by topics (left) and applications (right). The numbers represent the articles included in the respective category. In total, 130 articles were included. Two papers were sorted in two categories since the articles dealt with various topics and/or applications
Scientometric analysis: Research networks
The majority of journal articles were published by first authors who were affiliated with institutions in Asia (Fig. 5), particularly in China (n = 27, 20.8%), Thailand (n = 23; 17.7%), Malaysia (n = 18; 13.8%), Indonesia (n = 12, 9.2%), and Japan (n = 11; 8.5%). Besides, outside of Asia authors with affiliations in Australia (n = 6, 4.6%) and Germany (n = 4; 3.1%) were majorly present. The seven papers that were not included in the study, because they were not written in English were published in Japanese (n = 4), Chinese (n = 2) and Bahasa Indonesia (n = 1).
In the following, the main output articles grouped in the topics of “Ecophysiology”, “Biochemical composition” and “Water treatment” with the respective applications of “Cultivation”, “Nutritional value” or “Post-harvest” were summarized by topics.
Ecophysiology
A total of 20 papers were grouped under the topic “Ecophysiology” with 17 articles conducting research on the topic of “Ecophysiology” and the application of “Cultivation” (n = 9), followed by “Post-harvest” (n = 6) and “Nutritional value” (n = 2, Figs. 4, 6). The studies focused on the effect of one or more abiotic parameters on the physiology of the alga, and light was the major parameter studied (n = 8, Fig. 6A). The response variables seemed to depend on the application (Fig. 6B), with biomass production, biochemical composition and chlorophyll a fluorescence being most commonly used for research in the topic of “Cultivation”, whereas studies focusing on “Post-harvest” mainly quantified water content, biochemical composition and colour/ pictures (Fig. 6B). Most studies were designed to test the effect of a single factor (n = 13), rather than running a crossed design experiment (n = 4, Fig. 6C).
Count of A experimental parameters tested, with alternating current electric field abbreviated as ACEF, B response variables quantified, C experimental design and D seaweed status performed in papers in the topic of “Ecophysiology”, “Nutritional value” and “Post-harvest”, grouped by the different applications (Count of papers: Cultivation (n=9), Nutritional value (n=2), Post-harvest (n=6))
Cultivation
Biomass production (n = 6) and the biochemical composition (n = 6), including especially pigment (Guo et al. 2015a, b; Kang et al. 2020; Cai et al. 2021b), protein (Long et al. 2020b; Cai et al. 2021b) or fatty acid content and pattern (Long et al. 2020b), were the most frequently used response variables (Fig. 6B, On-line Appendices III), whereas light was majorly used as an experimental parameter (n = 5, Fig. 6A). The shade-adapted sea grapes showed highest biomass productions at photosynthetically active radiations (PAR) of 40 and 100 µmol photons m−2 s−1, compared to 20 and 100 µmol photons m−2 s−1 (Guo et al. 2015b) and 50 and 150 µmol photons m−2 s−1 (1Blue, 5Red light emitting diodes/LED; Kang et al. 2020), respectively. However, irradiances of ≥ 100 µmol photons m−2 s−1 were reported to cause physiological stress (Guo et al. 2015b; Kang et al. 2020; Stuthmann et al. 2020). Besides the level of irradiances, which also impacted sea grapes´ morphology (Guo et al. 2015b; Fakhrulddin et al. 2021), the photoperiod and the light spectrum had profound effects on the physiology of sea grapes, resulting in different biomass productivities, pigment contents and bioactivities (Kang et al. 2020). Blue light triggered phytoene desaturase (PDS) expression and antioxidant activities, whereas red light rather enhanced biomass production. Hence, authors recommended a spectrum of 1B5R (16.7% blue + 83.3% red) and a photoperiod of 12 h light and 12 h darkness for the indoor cultivation of sea grapes (Kang et al. 2020). UV light is known to induce oxidative stress in seaweeds (Dring 2005) and a reaction of C. lentillifera to different exposure scenarios is to be expected. However, only low absorbance in the UV-spectrum was recorded for sea grapes (Tanaka et al. 2020) and the effect of UV light on the ecophysiology of C. lentillifera has not yet been investigated. Despite light being the main experimental stressor, the studies were only running for 7 days until 4 weeks and only one article focused on shorter exposure times (< 72 h, On-line Appendices III; Terada et al. 2021).
Three studies focused on the effects of temperature, salinity, and nutrients on the physiology of C. lentillifera, respectively (Fig. 6A, Table 1). Temperature and salinity had profound effects on the biomass production of sea grapes, with highest growth rates at 27 °C and 27.5 °C (at 60 and 40 µmol photons m−2 s−1, respectively; Guo et al. 2015b; Cai et al. 2021b) and 35 PSU (Guo et al. 2015a; Tanaka et al. 2020). Temperatures and salinities outside of the optimal conditions caused not only a decrease in biomass production, but also changes in the photosynthetic efficiency Fv/Fm, photosynthesis vs. irradiance curve parameters, enzymatic antioxidant expression (catalase/CAT, superoxide dismutase/SOD) and pigment content (Table 1). With regards to nutrients, the effect of nitrate (Guo et al. 2015a; Cai et al. 2021b) and phosphate levels (Guo et al. 2015a), as well as different fertilizers (Fakhrulddin et al. 2021) on the physiology and growth of sea grapes were tested. Nitrate levels did not affect the growth rates of sea grapes at phosphate levels of 10, 29, and 400 µmol L−1 (Guo et al. 2015a; Cai et al. 2021b), respectively; whereas an effect was reported at 100 µmol L−1 of phosphate (Guo et al. 2015a). Consequently, the presence of commercial fertilizers did result in higher biomass production, compared to the control with natural sea water (Fakhrulddin et al. 2021). Increasing nitrogen (N) levels lead to ascending chlorophyll a, carotenoid and soluble protein concentrations (Guo et al. 2015a; Cai et al. 2021b), which might however also depend on the prevailing phosphate concentrations (Guo et al. 2015a). Nutrient accumulation of C. lentillifera seemed also influenced by the presence of bottom sediment, which caused an increase in ash, mineral elements and heavy metals, and changes in the amino acid composition; but decreased the content of PUFAs and carbohydrates (Long et al. 2020b). Five studies investigated the effect of a single parameter, while four quantified cross effects (Fig. 6C, Table 1). Interactive effects of light and temperature (Guo et al. 2015b; Terada et al. 2021), phosphorous (P) and N concentrations (Guo et al. 2015a), and N levels and temperature (Cai et al. 2021b) were studied. For instance, effects on photosynthesis and respiration of C. lentillifera caused by temperature were reversed by increases in the nitrate level, implicating that eutrophication and climate change could have interactive effects on sea grapes during cultivation (Cai et al. 2021b).
Post-harvest
Six papers investigated the ecophysiology of C. lentillifera with a focus on their post-harvest. Four of these focused on the post-harvest storage of the fresh seaweed product in different packaging environments, where desiccation was the main stressor. Therefore, the authors most frequently chose water content as the essential response variable (Fig. 6A, B; Terada et al. 2018; Stuthmann et al. 2020; Liang et al. 2021; Sulaimana et al. 2021).
Desiccation of C. lentillifera fronds, independent of the different packaging materials and experimental set-ups, resulted in varying degrees of water loss over different experimental runs, from ~ 5% after 5 days (Liang et al. 2021), to ~ 25–40% after 9 days (Sulaimana et al. 2021) and ~ 9 to 72% water loss after 12 days (Table 1; Terada et al. 2018; Stuthmann et al. 2020). Desiccation induced oxidative stress, quantified by decreasing Fv/Fm values (Terada et al. 2018; Stuthmann et al. 2020; Liang et al. 2021), increasing levels of stress biomarkers, including malondialdehyde (MDA), superoxide anion, hydrogen peroxide, peroxidase, proline and antioxidant enzymes (CAT, SOD, peroxidase/PER) (Liang et al. 2021; Sulaimana et al. 2021), as well as decreases in chlorophyll a, b and soluble protein content (Liang et al. 2021; Sulaimana et al. 2021). However, when sea grapes were rehydrated after desiccation, a recovery, e.g. by increasing photosynthetic efficiency values (Fv/Fm) was documented (Terada et al. 2018; Stuthmann et al. 2020; Liang et al. 2021). Supra-optimal light irradiances of PAR induced additional stress on the physiology of sea grapes, resulting in higher Fv/Fm decreases and the trend of colour loss (Stuthmann et al. 2020). Hence, room irradiances (3 µmol photons m−2 s−1) were suggested during sea grape storage (Stuthmann et al. 2020). Sea grapes are commonly stored for transport or retail in plastic containers, however, the plastic material differs and seemed to affect the physiological response of the seaweed (Terada et al. 2018; Stuthmann et al. 2020). Additionally, the initial constitution of the sea grapes, e.g. influenced by the harvesting season, had an effect on the physiological response during desiccation, with slower decomposition rates at better initial physiochemical constitutions (Sulaimana et al. 2021). Additionally, applying alternating current electric field (ACEF) on C. lentillifera to suppress reactive oxygen species (ROS) accumulation during storage resulted in reduced water loss, chlorophyll and phenol degradation, as well as MDA production and thus provides a post-harvest treatment method with potential that should be further investigated (Sulaimana et al. 2021).
Two studies investigated the ecophysiology of sea grapes which were not alive (Fig. 6D), but cured in a brine solution and oven-dried (Anantpinijwatna et al. 2018) during post-harvest to extend the shelf-life (Tolentino et al. 2021). Storage in brine solutions ≤ 5% exceeded the bacterial limit and was rated less acceptable in sensory testing, whereas storage in brine solutions of 10, and 15% for ten days had acceptable bacterial counts and better sensory evaluations, regarding e.g. colour, odour and texture, especially after re-hydration (Tolentino et al. 2021). However, total chlorophyll and carotenoid content decreased significantly more at higher salinity concentrations (Tolentino et al. 2021).
Nutritional value
The nutritional value was the focus of two studies from the topic “Ecophysiology”. The antioxidant activity was triggered by exposure of sea grapes to light-stress and resulted in higher antioxidant activity values of light-stressed algae (300 µmol photons m−2 s−1, 14 days), compared to the dehydrated product and similar values to that of the renowned “super fruit” pomegranate (Sommer et al. 2022). However, the chlorophyll content decreased during light-stress exposure, causing a bleaching of the alga and potentially decreasing consumer acceptance (Stuthmann et al. 2022). Therefore, the duration and intensity of the light treatment should be applied to the intended usage of the biomass as a fresh (e.g. food product) or dry (e.g. cosmetic) product. Medium irradiances (200–600 µmol photons m−2 s−1) and shorter exposure periods (3–7 days) resulted in significantly enriched antioxidants, but without strong bleaching of the sea grapes, whereas high irradiances and longer exposure periods (200–600 µmol photons m−2 s−1, up to 14 days) increased antioxidants even more, but with significant loss of chlorophyll and colour (Stuthmann et al. 2022).
Biochemical composition
The topic “Biochemical composition” comprised the overall highest number of papers, compared to the other topics (Fig. 3A), with the applications of “Nutritional value” (n = 16), “Post-harvest” (n = 3) and “Cultivation” (n = 3) accounting for a total of 23 (Fig. 4). The authors focused on different biochemical compounds and hence conducted various analyses (Fig. 7, On-line Appendices IV). Considering all three applications, proximate analysis (n = 9) and antioxidant activity (n = 9) were analysed most frequently, followed by mineral (n = 8) and fatty acid analysis (n = 7) and the total phenolic content (n = 6, Fig. 7A). Most of the studies compared C. lentillifera (intra-species comparisons, n = 10) from different regions (Zhang et al. 2020), cultivation seasons (Wichachucherd et al. 2019) or set-ups (Syamsuddin et al. 2019), whereas nine and six studies compared C. lentillifera with species from a different (inter-species comparison) or the same genus (intra-genus comparison, Fig. 7B).
Count of A analyses and B comparison with species of other genus (inter-species), other species from the genus Caulerpa (intra-genus) and comparisons of Caulerpa lentillifera (intra-species) performed in papers in the topic “Biochemical composition”, grouped by the different applications (Count of papers: Cultivation (n=2), Nutritional value (n=16), Post-harvest (n=3))
Cultivation
Two intra-species comparisons quantified seasonal (Wichachucherd et al. 2019) and cultivation method (Syamsuddin et al. 2019) related effects on the biochemical composition of sea grapes in the frame of their “Cultivation” (Table 2, Fig. 4). Indoor and outdoor cultivation of C. lentillifera resulted in biochemical differences in sea grape tissue, possibly due to sediment type and light irradiances. Caulerpa lentillifera showed differences in mineral (indoor: 49.92—52.79% ash, outdoor: 32.04 – 36.60% ash), carotenoid (indoor: 1.32—2.11 ppm, outdoor: 1.71—2.29 ppm) and fibre (indoor: 5.03—5.56%, outdoors: 7.64—8.65%) content, as well as weight increase (indoor: 1.17 – 80.12 g, outdoor: 1.66 – 25.15 g; Syamsuddin et al. 2019). However, substrate mixture, culture depth (Syamsuddin et al. 2019) and temporal changes in salinity and nitrate content (Wichachucherd et al. 2019) also caused differences in specific target substances.
Nutritional value
The edibility of sea grapes was the most common reason given for their research by scientists investigating the “Nutritional value” (Table 2). The components examined seemed to follow a pattern. Analysis of proximate composition was often conducted in combination with the fatty acid and amino acid content, vitamins and minerals (Ratana-arporn and Chirapart 2006; Salleh and Wakid 2008; Matanjun et al. 2009; Zhang et al. 2020). Researchers also focused on the antioxidant composition, namely the antioxidant activity, total phenolic content and/or total flavonoid content (Matanjun et al. 2008) in combination with minerals (Nufus et al. 2019; Ismail et al. 2020), pigments (Balasubramaniam et al. 2020), or the proximate composition (Nguyen et al. 2011;Table 2).
The authors quantified mean water contents ranging from 87.05 – 95.95% fresh weight (FW). Sea grapes were rather high in carbohydrates (27.19—72.90% dry weight/DW) and crude proteins (9.26—19.38% DW), but lower in crude lipids (0.70—2.87% DW) and fibre (1.91—12.98% DW). The mean ash content ranged widely (2.10—47.80% DW), which might have influenced the equally wide range of minerals (On-line Appendices V). Overall, highest concentrations of macro- and microminerals were found for sodium (Na, 1229.7—16,050 mg (100 g)−1 DW) and iron (Fe, 9.3—1972.9 mg (100 g)−1 DW), respectively (On-line Appendices VI). Regarding the fatty acid composition, C. lentillifera contained mostly saturated fatty acids (SFAs, 40.7—82.69% of total fatty acids) and approximately similar amounts of mono-unsaturated fatty acids (MUFAs, 8.43—36.83% of total fatty acids) and PUFAs (9.49—38.07% of total fatty acids). Palmitic acid (C16:0, 8.74 – 49.46% of total fatty acids), omega-6 PUFA Linoleic acid (C18:2N6C, 4.26—11.85% of total fatty acids) and omega-3 PUFA α-Linolenic (C18:3N3, 2.73—13.42% of total fatty acids) were most abundant (On-line Appendices VII). The total amino acids (101.63—147, with for human essential 44.02—57.01 and non-essential 54.08—89.99 mg g−1 DW) were mainly represented by the essential amino acids glutamic acid (Glu, 13.47 -17.8 mg g−1 DW), aspartic acid (Asp, 8.33—14.89 mg g−1 DW) and glycine (Gly, 5.14—19.23 mg g−1 DW) and the non-essential valine (Val, 6.18—11.16 mg g−1 DW), leucine (Leu, 7.79—12.86 mg g−1 DW) and phenylalanine (Phe, 4.81—19.95 mg g−1 DW, A VIII). Lysine (1.22—8.2 mg g−1 DW) was reported to be the most limiting amino acid in C. lentillifera (Matanjun et al. 2009; Terriente-palacios and Castellari 2022).
The total phenolic content of C. lentillifera was quantified using the Folin-Ciocalteu (FC) assay and ranged from 1.30 to 57.97 mg gallic acid equivalents[GAE] g−1 DW (On-line Appendices IX, Matanjun et al. 2008; Nguyen et al. 2011; Ismail et al. 2020). The total flavonoid content was only determined once (1506.41 mg Quercetin equivalent (100 g)−1; Ismail et al. 2020). Different assays with individual sets of (dis)advantages (Karadag et al. 2009) were often used supplementary and correlations between the results were common (Matanjun et al. 2008).
Pigment composition was quantified by two studies, whereas one only focused on chlorophyll a and b (258 ± 25 and 147 ± 14 mg (100 g) −1 DW) and the other one on a variety of others, including canthaxanthin, and astaxanthin. Both studies also determined β-carotene (15 ± 1.0 and 19.5 ± 0.0 mg g (100) −1 DW) content (On-line Appendices X). The vitamin contents of vitamins A, B1(thiamine), B2 (riboflavin), B3 (niacin), C (ascorbic acid), and E (alpha-tocopherol) were investigated in four studies. Vitamin C (1—50.33 mg (100 g) −1 WW) was the most prominent vitamin, followed by E (2.22—8.41 mg (100 g) −1 WW). However, B vitamins 1, 2, 3 were present in concentrations < 1.1 mg (100 g) −1 WW (On-line Appendices XI). Vitamin D content has not yet been quantified, even though the vitamin was found in other (green) seaweeds (Debbarma et al. 2016). The majority (n = 9) of studies conducted an inter-species comparison of C. lentillifera with seaweeds from genera outside of Caulerpa, followed by six studies comparing the alga with other Caulerpa species and five studies investigating only C. lentillifera (Fig. 7B).
Authors stated that they chose the seaweeds based on their presence at the study location, and often also due to their role in human nutrition. Hence, Sargassum and Eucheuma were most prominent, besides C. lentillifera (Table 2; Matanjun et al. 2008, 2009; Salleh and Wakid 2008; Balasubramaniam et al. 2020). In the direct comparison with these seaweeds, C. lentillifera showed significantly enriched carbohydrate, sodium, magnesium, and total amino acid contents, but was significantly depleted e.g. in ash, crude fibre, and iodine (Matanjun et al. 2009). On the other hand, C. lentillifera had by far the highest ash content when compared to Chaetomorpha, Gracilaria and Ulva (Setthamongkol et al. 2015). The vitamin contents were in a similar range with Sargassum and Eucheuma species (Salleh and Wakid 2008; Matanjun et al. 2009), but seemed to be higher compared to Ulva reticulata (Ratana-arporn and Chirapart 2006). Besides, the PUFA content of C. lentillifera was significantly lower compared to E. cottonii and S. polycystum (Matanjun et al. 2009). Homotaurine and hypotaurine contents of C. lentillifera (0.60 ± 0.05, 0.14 ± 0.02 mg (100 g)−1 DW), as well as the essential to non-essential amino acid ratio (0.51 ± 0.02) were compared to a variety of other commercial seaweed products (Terriente-palacios and Castellari 2022). The total phenolic content and antioxidant activities (ABTS, FRAP) were enriched compared to E. cottonii and E. spinosum (22.50 ± 2.78, 15.82 ± 1.24 mg phloroglucinol equivalents[PGE] g−1 DW) and other red and brown seaweeds of the genera Dictyota, Padina and Halymenia (Matanjun et al. 2008). On the other hand, radical scavenging activity (DPPH assay) and antioxidant activity (ORAC) of sea grapes were lower than in Eucheuma denticulatum (Balasubramaniam et al. 2020).
In the intra-genera comparison, C. lentillifera was mostly investigated alongside C. racemosa (Table 3; Matanjun et al. 2008; Salleh and Wakid 2008; Nagappan and Vairappan 2014; Paul et al. 2014; Setthamongkol et al. 2015; Ismail et al. 2020), but the results did not show a clear trend and seem to be highly influenced by local factors. Thus, C. lentillifera was reported to have overall lower nutritional values than C. racemosa regarding the PUFA and pigment content (Paul et al. 2014), but also with higher PUFA content (Nagappan and Vairappan 2014). The total phenolic content values of both Caulerpa species were similar (C. lentillifera 42.85 ± 1.22 vs C. racemosa 40.36 ± 1.05 mg PGE g−1 DW; Matanjun et al. 2009), however, the growth rates were unanimously reported significantly higher for C. lentillifera (Paul et al. 2014; Setthamongkol et al. 2015). Intra-species comparisons were conducted in order to test for the effect of growth region (Zhang et al. 2020), cultivation set-up (laboratory, the wild, or mariculture; Shevchenko et al. 2009; Saito et al. 2010) or different extraction and analytical methodologies (Nguyen et al. 2011; Long et al. 2020a). Zhang et al. (2020) found among others significant differences in the proximate composition, and vitamin C content in C. lentillifera from China´s Hainan and Shandong province. However, the cultivation set-up (Shevchenko et al. 2009; Saito et al. 2010), and the analytical methodology also had an effect on the nutritional composition. Thermal drying yielded significantly lower phenolic contents, compared to freeze drying (1.30 ± 0.02 vs 2.04 ± 0.03 mg GAE g−1 DW; Nguyen et al. 2011).
Post-harvest
Three studies investigated the “Biochemical composition” of sea grapes within the application of “Post-harvest” (Fig. 4), all aiming to contribute to a circular economy approach by valorising waste biomass of C. lentillifera generated during the aquaculture. The nutritional value was reported to be not different from that of food-grade products (Chaiklahan et al. 2020). The studies focused on the polysaccharide (Chaiklahan et al. 2020; Honwichit et al. 2022), as well as the lipid (Srinorasing et al. 2021) fraction. In intra-species comparisons (Table 2) different extraction methods, namely varying algae-to-ethanol ratios, extraction times, stages and purifications were tested to obtain the highest yield of the respective target metabolites (Chaiklahan et al. 2020; Srinorasing et al. 2021). Regarding polysaccharide extraction, two-stage extraction with 60 min/stage, a solid-to-liquid ratio of 1:15 (w/v), extraction temperature of 90 °C, and two time precipitation by a concentration of 75% ethanol was reported to obtain highest polysaccharide yields of around 25% of DW (Chaiklahan et al. 2020) and the hot water extraction (pH 6, 90 °C for 20 min) was the most cost-effective (Honwichit et al. 2022). For lipid extracts, optimum extraction conditions were three-stage extraction with 15 min/stage, solid-to-liquid ratio of 1:10 (w/v) at room temperature for 30 min. At these conditions, crude lipids yields of around 28% DW were obtained (Srinorasing et al. 2021). One study conducted an economic evaluation for the production of polysaccharide in Thailand (Chaiklahan et al. 2020). Based on their estimation the polysaccharide extract could be profitable for the farmers.
Water treatment
Within the topic of "Water treatment”, papers were grouped into the applications “Industrial effluents” (n = 10), “Cultivation” (n = 11), and “Nutritional value” (n = 2), of which only the latter two were evaluated here.
Snails, fish, and shrimp were most often co-cultivated with C. lentillifera, followed by other seaweeds (Fig. 8A). The most prominent treatment and response variable quantified were the mono- vs co-culture applied by seven studies (Fig. 8B) and the nutrient removal/uptake rate (n = 9, Fig. 8C).
Cultivation
A total of eleven studies focused on the application of “Cultivation” within the topic of “Water treatment”. The majority were conducted in pilot aquaculture systems (n = 8), including e.g. experimental recirculating aquaculture systems (RAS), open water integrated multi-trophic aquaculture (IMTA), or larger scale same tank cultures, whereas three studies were conducted at laboratory scale (Table 3). The experiments conducted in larger-scale systems had considerably longer experimental runs (19 – 120 days, mean: 58 days), compared to the laboratory-based studies (10 h, 24 h and 15 days, Table 3, On-line Appendices XII).
The majority of studies focused on C. lentillifera as a bioremediator of nutrients in aquaculture effluents from different organisms (Table 3), whereas one study investigated the ability to remove sterol hormones (Lu et al. 2021). Caulerpa lentillifera was mostly integrated with one other species. Snails (Babylonia areolata, abalone) and fish (Poecilia latipinna, Lates calcarifer and grouper) were the most prominent organisms co-cultured with sea grapes, followed by shrimp (Litopenaeus vannamei), sea cucumber (Holothuria scabra) and sea urchin (Table 3, Fig. 8A). However, even though abalone were investigated in two studies (Haliotis asinine: Largo et al. 2016; species unknown: Paul et al. 2014), reports on its compatibility for co-culture with C. lentillifera are still missing. On the one hand, C. lentillifera was reported to be too fragile for the culture in baskets as part of an open water IMTA system with abalone, and therefore had to be replaced with a more robust species (Largo et al. 2016). On the other hand, although Paul et al. (2014) used abalone and sea urchins as cultivation medium, the authors focused on the co-cultivation of two Caulerpa species and did not further report on the fed-species.
The biomass production, usually expressed as growth rate, ranged from 0.46 – 4% day−1 (On-line Appendices XII). On the one hand, C. lentillifera showed higher growth rates when compared to C. racemosa (Paul et al. 2014) and Gracilaria salicornia (Chaitanawisuti et al. 2011). On the other hand, similar and lower values were obtained when compared to G. lichenoides (Liu et al. 2016) and other Caulerpa species (Paul and de Nys 2008). Besides biomass production, the texture of sea grapes is a unique selling point (de Gaillande et al. 2017) and therefore, biomass properties, including the respective frond to stolon ratio, the harvestable biomass, as well as the ramuli density are important parameters to consider, so far reported by two studies (Paul et al. 2014; Dobson et al. 2020). Most studies experimentally compared the mono-culture of fed-species with the integration of C. lentillifera (mono- vs. co-culture, Fig. 8B), reporting positive effects on the water quality and the fed-species (Table 3).
The nutrient removal/uptake was the most prominent response variable (n = 8, Fig. 8C), highlighting the function of the seaweed in the co-culture set-ups as a bioremediator. Various studies reported that C. lentillifera efficiently removed nutrients from aquaculture effluents, leading to decreased N (total ammonia, nitrite, nitrate; Dobson et al. 2020) and P levels (Chaitanawisuti et al. 2011; Bambaranda et al. 2019a, b; Anh et al. 2021; Ly et al. 2021), also recognizable by negatively correlated nutrient loads with sea grape densities (Anh et al. 2021; Ly et al. 2021; Margono et al. 2021) and N-enrichment in sea grape tissue (Paul and de Nys 2008; Liu et al. 2016; Bambaranda et al. 2019b). Sea grape tissue-N was one of the quantified response parameters summarized as biochemical composition, along with tissue-C content (Paul and de Nys 2008; Liu et al. 2016), chlorophyll (Lu et al. 2021), and heavy metal content (Bambaranda et al. 2019b). The growth of C. lentillifera was higher in a low N environment (0.017 mg L−1, ~ 3% day−1), compared to a high N environment (1.4 mg L−1, ~ 4.2% day−1; Paul and de Nys 2008) and at ammonia:nitrate ratios of around 1:5, as the species seemed to prefer nitrate over ammonia as a N source, in the presence of both (Liu et al. 2016). As the nutrient load entering an aquaculture system mainly depends on the fed-species, it is not surprising that the growth rate of sea grapes was also affected by the feeding rate and density of L. vannamei in the same tank (Fig. 8B; Anh et al. 2021). The growth rates increased from 0.46 to 1.05% day−1 with increasing feeding rates, but decreased (0.45 – 0.82% day−1) with increasing shrimp densities (1000—3000 ind. m−3), possibly influenced by shrimp grazing on C. lentillifera (Anh et al. 2021).
However, in other cases, the growth performance and biomass properties of sea grapes were independent of the presence of the co-cultured species, such as for snails and sea cucumbers (growth rate: 1.86 ± 0.12% day−1; Dobson et al. 2020). These results indicated that the relation between nutrient input (feeding, stocking densities), water volume and algal biomass is essential, which can also be altered by adapting the initial seaweed stocking density in the system, as reported in three studies (Fig. 8B; Chaitanawisuti et al. 2011; Bambaranda et al. 2019a; Ly et al. 2021). Interestingly, the positive effect of the presence of C. lentillifera on growth rate, survival, and production of L. vannamei shrimp (Ly et al. 2021), as well as slight improvement of the yield and survival rate of snail B. areolata did not or only minimally depend on the initial C. lentillifera stocking densities (investigated ranges of 0.5 – 2 kg m−3; Ly et al. 2021 and 0.280 – 0.840 kg m−3; Chaitanawisuti et al. 2011). However, C. lentillifera growth rates significantly decreased with increasing initial biomass (Chaitanawisuti et al. 2011; Ly et al. 2021), e.g. from 2.58 ± 0.09% day−1 at a density of 390 g m−2, to 1.92—1.70% day−1 at higher initial densities (790 g m−2, 1170 g m−2; Chaitanawisuti et al. 2011). Additionally, the growth tended to be highest in the first 14 – 20 days of the longer experimental runs, compared to the subsequent periods (Bambaranda et al. 2019b; Ly et al. 2021). This is most likely caused by changes in the light environment, due to increasing mutual shading of the algae (Bambaranda et al. 2019b) or loss of water transparency (Ly et al. 2021). Besides, feeding on C. lentillifera by L. vannamei may have led to improved food conversion ratios (FCR) of the shrimp, but also to decreases in sea grape biomass (Anh et al. 2021).
Same tray co-culture with C. racemosa resulted in lower biomass productivities regardless of the initial stocking densities, potentially due to a delayed establishment, suggesting rather a mono-culture of the species (Paul et al. 2014). On the other hand, the presence of sea grape trays was assumed to result in a nearly 50% decreased yield of sea cucumber Holothuria scabra, compared to the set-up without the seaweed. Arguably because the shading provided by the trays inhibited the growth of microalgae in the sediment, which are an essential food source of sea cucumbers (Dobson et al. 2020). Only one study conducted an economic assessment (Dobson et al. 2020), reporting that the integration of C. lentillifera could increase the gross yield value substantially (US$ 44.27 m−2), compared to a sandfish mono-culture (US$ 3.80 per m−2) and a sea cucumber – Babylonia (US$ 21.53 per m−2) system. The monetary yields were only based on the farm-gate prices and neglect the initial investment, as well as work-force (Dobson et al. 2020).
Apart from nutrients, C. lentillifera can also be used to effectively remove steroid hormones from grouper aquaculture effluents (Lu et al. 2021), which was investigated by changing the sterol content in the water and quantify the sterol uptake rates (Fig. 8B, C). Of the four investigated seaweeds (Ulva pertusa, G. lemaneiformis, and Codium fragile), C. lentillifera was most efficient in removing steroid hormones 17β-estradiol and 17α-ethinylestradiol (EE2) within 12 h (4 g L−1 seaweed, more than 90% removal). The sterol removal rates were also affected by temperature and salinity (Lu et al. 2021). Salinity and aeration affected nutrient uptake rates of C. lentillifera from effluents of a saline molly (Poeicilia latipinna) in a 24 h laboratory study, and the authors identified optimal salinity levels (29—30 PSU) and aeration regime (to be present) using (non)linear regression (Bambaranda et al. 2019a), before testing the set-up (30 g L−1, 30 PSU, aeration) in a scaled-up system (Bambaranda et al. 2019b). Due to substantial losses in an in-situ settlement pond experiment of a commercial barramundi (Lates calcarifer) aquaculture, possibly induced by epiphytic filamentous algae, the influence of fragment size and culture depth was tested. Depth did not affect C. lentillifera growth, but larger fragments (60.3 ± 10.6 g) seemed to induce higher losses compared to fragments one decimal smaller (6.4 ± 1.3 g; Paul and de Nys 2008).
Nutritional value
Two studies in the topic of “Water treatment” focused on the nutritional value of C. lentillifera. Both studies evaluated the co-cultivation of sea grapes and L. vannamei in the same culture unit with capacities of 50 L (Omont et al. 2022) and 500 L (Anh et al. 2022), respectively (Fig. 8A). The studies tested the effect of a shrimp and/or sea grapes mono- vs. co-culture (eight shrimp and 15.23 ± 0.02 g sea grapes, respectively; Omont et al. 2022) and different L. vannamei densities (100—500 ind. m−3 and 1 kg m−3 sea grapes; Anh et al. 2022) on sea grape biomass production, biochemical composition, including proximate composition (Anh et al. 2022; Omont et al. 2022) and mineral content (Omont et al. 2022), as well as nutrient removal efficiency (Fig. 8C; Omont et al. 2022). The presence of shrimp significantly increased the percentage (DW) content of protein, lipids and ash, while decreasing the carbohydrates, compared to the initial biomass retrieved from pond cultivation. However, increasing shrimp densities significantly increased the protein content and decreased the ash content of sea grapes, with fibre, moisture, carbohydrates and lipids being similar among the density treatments (Anh et al. 2022). On the other hand, Omont et al. (2022) also reported an increased percentage (DW) of protein for sea grape tissue in co-cultivation, but relatively lower ash contents. However, the total content of trace elements increased significantly (Na by 12.5%, molybdenum by 78.0%, boron by 50.8%), whereas the content of cobalt decreased (Omont et al. 2022). The growth of sea grapes was highly negatively affected by the presence of shrimp (40.6 ± 9.8 vs 2.6 ± 0.4% day−1; Omont et al. 2022) and tended to decrease with increasing shrimp densities, with significant depletion at the highest density treatment (500 ind. m−3, 1.30 ± 0.11% day−1; Anh et al. 2022). Grazing might have been a reason for this pattern, but it resulted in increased levels of iron (Fe) and zinc (Zn), total body cholesterol and muscle lipid content in the shrimp (Omont et al. 2022).
Discussion
Main research topics, applications, and author affiliation
Sea grape aquaculture has its roots in The Philippines and Japan (Okinawa) in the 1980s (Trono and Toma 1993; Estrada et al. 2021). Reliable, global production statistics for Caulerpa seaweeds are missing and local data are scarce, as they are not listed in national aquaculture statics (Moreira et al. 2021). However, it is generally accepted that the production and demand for this species is rising since approximately a decade ago. This might explain the elevated number of articles since 2018 revealed in the scientometric analysis, with > 60% of all publications (Scientometric analysis: Number of publications, research topics and applications). First authors were mainly from Asian countries (Scientometric analysis: Research networks) where the majority of sea grape cultivation takes place (Chen et al. 2019). The dominance of the research topic “Biochemical composition” and the application in “Pharmaceutics” highlights the interest in C. lentillifera for its bioactive compounds (Scientometric analysis: Number of publications, research topics and applications). Considering that marine natural products isolated from Chlorophyta are still underrepresented in the database MarineLit with 8% (1965–2012) compared to red (53%) and brown (39%) algae (Leal et al. 2013; Moreira et al. 2021), a further growth of interest in this topic and application is to be expected. This trend is possibly also driven by the comparatively higher values of these bioactive compounds in pharmaceuticals or cosmetics, compared to biomass e.g. for animal feed and biopolymers of feedstock (Chopin and Tacon 2021).
Sea grapes and their (a)biotic environment
Several environmental parameters are important for the cultivation of C. lentillifera. They can be adjusted precisely to the seaweeds´ needs during indoor cultivation, which is, however, associated with higher effort compared to outdoor cultivation. For the outdoor cultivation, crossed and interactive effects of different environmental factors are particularly important, considering daily, seasonal, or long-term changes and shifts. In the Northwestern Pacific, temperature and irradiance were major factors limiting C. lentillifera cultivation to certain seasons (Terada et al. 2021), whereas in The Philippines and other South-East Asian regions, temperature and salinity, caused by precipitation during the rainy season, restrict the cultivation to the dry season (Estrada et al. 2021). The cross-effects of many of these factors on the seaweeds’ physiology and biochemical composition, like salinity and temperature, have not yet been tested in experimental set-ups, leaving room for further studies. Chemical diversity within a single seaweed species is not uncommon and spatial as well as temporal variability of environmental parameters are often cited as causes (Stengel et al. 2011). Different compounds are generally the result of specific responses to environmental parameters (Stengel et al. 2011). However, many of the studies evaluated in Nutritional value conducted inter-species or intra-genus comparisons of sea grapes´ nutritional value with other, often edible or economically interesting, seaweeds from the same location. In contrast, only a few studies intra-specifically compared biochemical composition of C. lentillifera across spatial regions, temporal scales, and culture methods (Cultivation, Nutritional value). However, the reported intra-specific variability enforced the importance of understanding the effects of single and crossed (a)biotic culture factors on the physiology (Cultivation), microbial community (Topic: "Microbiome", Application: "Cultivation"; Pang et al. 2022), and biochemical composition (Nutritional value; Wichachucherd et al. 2019) of the sea grapes observed in the pond environment. Besides, chemical variability between thallus parts is common within seaweeds (Stengel et al. 2011), and should be investigated for C. lentillifera, especially since differential gene expression in the thallus parts have been reported (Arimoto et al. 2019b).
The special role of light
Light has been identified as a major stressor for sea grapes, due to their unusually low irradiance saturations, also compared to other green seaweeds (e.g. Codium spp., Ulva spp.; Nakamura et al. 2020; Marques et al. 2021). Hence, supra-optimal irradiances induced oxidative stress during cultivation (Cultivation) and post-harvest (Nutritional value), but they were also reported as an opportunity to increase the nutritional quality of C. lentillifera, by triggering its antioxidant production (Nutritional value). Sub-optimal irradiances, on the other hand, might have caused decreases in growth rates after a certain cultivation period, as reported from pilot co-culture systems (Cultivation). Consequently, management of initial biomass or harvest periods could ensure continuously optimal light conditions or purposefully increase sea grapes’ quality already in the culture set-up (Magnusson et al. 2015). Most studies focused on the continuous exposure to PAR with consistent photoperiods (Water treatment) and only individual studies included changes in absorption spectra for photosynthesis, photoperiod, and short or extended exposure times (Kang et al. 2020; Terada et al. 2021). This leaves various knowledge gaps for further studies, such as considering variations on the temporal continuums, from high frequencies (e.g. evoked by high turbidity, movement of the cultivation covers, passing of co-cultured species), medium frequencies (daily solar cycle) to low frequencies (seasons; Comerford et al. 2021). Additionally, even though C. lentillifera seemed to contain only minor quantities of UV-absorbing compounds (Tanaka et al. 2020), the exposure to this stressor could impact the physiology of the alga and alter the secondary metabolite composition, as observed for other seaweeds (Polo and Chow 2022).
Sea grapes (not only) as bioremediators in co-culture approaches
The nutrient acquisition of seaweeds is complex and depends on various parameters (Roleda and Hurd 2019). The N and P loads in the application of cultivation water were reported to affect biomass production and composition of sea grapes in both mono-, and co-culture (Cultivation and Cultivation). Sea grapes bioremediated nutrients from the water, as indicated by reduced water nutrient levels (Cultivation). However, the actual nutrient acquisition rate was only examined once in the context of preferred N-sources (Liu et al. 2016). The comparison between studies focusing on the nutrient uptake was difficult, as different thallus parts of C. lentillifera were used. Fronds and the below ground parts (stolon with rhizoids) are expected to have different N and P acquisition rates, as reported for C. prolifera (Alexandre and Santos 2020). This might explain the differences in composition, caused by the presence of bottom sediments and potentially higher nutrient loads in the pore water, compared to the water column (Long et al. 2020b). These information have important implications for the choice of cultivation method (trays, sowing method or the open water cage cultivation; Syamsuddin et al. 2019), especially when sea grapes are exposed to unusually high (e.g. for nutrient bioremediation in aquaculture; Cultivation, or eutrophication; Cai et al. 2021b) or rather low (oligotrophic waters) nutrient loads. Besides, salinity (Bambaranda et al. 2019a), N-sources (Liu et al. 2016) and potentially various other parameters (Roleda and Hurd 2019) affected the nutrient uptake rates. Higher growth rates than other Caulerpa species and the preference of nitrate over ammonia makes C. lentillifera a promising candidate for co-cultures (Cultivation). Regarding the application of sea grapes as a bioremediator in aquaculture systems with fed-species, it might be beneficial to implement polyculture of different seaweeds in order to remove different nitrogen compounds more efficiently. Commonly used species for biofiltration include Ulva lactuca and Undaria pinnatifida (Cahill et al. 2010), Gracilaria birdiae and Gracilaria vermiculophylla (Marinho-Soriano et al. 2009; Abreu et al. 2011), and Porphyra leucosticta which take up mainly ammonia (Chung et al. 2002). Since aquaculture effluents are usually higher in nitrate than ammonia content, implementing C. lentillifera together with commonly used species can enhance the bioremediation of the effluents (Neori et al. 2004). This has only been studied once (Paul et al. 2014). In general, C. lentillifera is a promising candidate for the use as a biofilter in integrated tank-based aquaculture systems, rather than in open water systems, at least when exposed to high water movements (Water treatment). However, when sea grapes were integrated in the same unit with L. vannamei, grazing of the shrimp on the seaweed was reported (Water treatment), leading on one hand to a loss of biomass, but on the other hand to reduced FCRs and a beneficial change in nutritional composition of the shrimp (Ly et al. 2021; Omont et al. 2022). Similarly, the integration of C. lentillifera powder in the feed (30 g kg−1) of the black tiger shrimp (Penaeus monodon) significantly increased growth rate and FCR of the post larvae (Topic: “Biochemical composition”, Application: “Animal feed”; Putra et al. 2019). However, a spatial segregation of the species could allow for a targeted feeding with sea grape biomass, e.g. the lower quality waste, integrated in the feed or provided fresh, and still allow for the seaweeds to bioremediate nutrients. On the other hand, this would require more space which could negatively impact the costs for the farmers (Dobson et al. 2020), reinforcing the importance of an economical assessment as basis for farmers decision making.
Economic assessment
Sea grape farming has been described as a lucrative business in The Philippines, among others, with the potential for global upscaling, but limited awareness has been identified as a hurdle (Dumilag 2019; Estrada et al. 2021). Ethnophycological studies are least represented in this literature review (Scientometric analysis: Number of publications, research topics and applications), even though the sea grape farmers are an essential part of the value chain of C. lentillifera, their knowledge, needs and access to scientific findings are of great interest. An essential part of such applied research could be the integration of an economic analysis of new co-culture approaches or cultivation and post-harvest methods, which has only been done scarcely (Chaiklahan et al. 2020; Dobson et al. 2020). The farm-gate price, likely for the use in human nutrition, of sea grapes reported from Vietnam (US$ 4.35 kg−1; Dobson et al. 2020) lies clearly above the rather low average value of brown, red and green seaweed biomass (US$ 0.47, 0.39, 0.79 kg−1 WW, respectively; Cai et al. 2021a). Hence, sea grape farming could provide a good source of income, especially as initial investments, e.g. in the tidal pond cultivation, are rather low. Considering that parts of the harvest do not meet the required quality standards (Chaiklahan et al. 2020), the waste valorisation should be brought into focus (Post-harvest).
Sea grapes as human food
Red and brown algae dominate the commercial seaweed production and the share of green macroalgae is vanishingly low (FAO 2020; Moreira et al. 2021). Caulerpa lentillifera can compete with commercial seaweeds regarding their nutritional value, exhibiting similar or even higher amounts of for example minerals, vitamins, and antioxidative properties (Cultivation). The biochemical composition, including protein, lipid and carbohydrate content and quality, as well as bioactive compounds over vitamins and pigments varied considerably between studies (Biochemical composition, On-line Appendices V—XII), supported by a recent review on health benefits and nutrients of C. lentillifera (Syakilla et al. 2022).
Since the amounts of different biochemical compounds showed a large variability, it is important to understand the factors leading to these differences in order to improve their concentrations in the framework of C. lentillifera as functional food ingredient. The cultivation conditions or set-ups could even be managed to increase certain target compounds, like antioxidants (Nutritional value) or proteins through co-cultivation (Nutritional value). One delicate part in the life-cycle of C. lentillifera is post-harvest handling, as the product is still alive and photosynthetically active. The shelf-life is therefore considerably short and quick transportation and retail are required. The packaging and storage materials differ locally, as well as the form of retail (de Gaillande et al. 2017), ranging from natural materials up to plastic (Terada et al. 2018; Stuthmann et al. 2020). The packaging materials (Terada et al. 2018; Stuthmann et al. 2020), in addition to the environmental conditions during previous cultivation (Minh et al. 2019) and during storage, influenced the quality of the sea grapes (Nutritional value; Stuthmann et al. 2020). However, considering studies on C. lentillifera during cultivation, it is expected that temperature (Terada et al. 2018), as well as the microbiome (Topic: “Microbiome”, Application: “Cultivation; Liang et al. 2019; Kopprio et al. 2021) also have a major impact on the quality of the sea grapes during storage. The main costumer base for C. lentillifera is currently in Asia. However, the interest in this food product might be growing in Europe as well, especially since the demand for vegetarian/vegan food products (Lusk 2017) and the awareness of health and environmental issues related to food choices (de Boer et al. 2007; Wendin and Undeland 2020) is increasing. Sea grapes, with their unique texture and nutritional components, are an interesting candidate to contribute to human nutrition outside the current market in Asia. However, this requires advances in the land-based cultivation of this tropical species or in the improvement of the shelf-life. Furthermore, C. lentillifera is not yet considered by the European Novel food law (Barbier et al. 2019; Mouritsen et al. 2019). While single brown and red seaweeds (e.g. representatives of the orders Laminariales, Fucales and of the genera Porphyra/Neopyropia) are included in the Novel Food Catalogue, edible green macroalgae were rather neglected (Lähteenmäki-Uutela et al. 2021).
Conclusions
Caulerpa lentillifera is a promising candidate for aquaculture in general and for co-cultivation, especially since the value of the product is higher compared to other seaweeds, among others due to its striking texture (“green caviar”). The present review highlighted the interest in the alga’s “Biochemical composition” with the application for “Pharmaceutical” and “Nutritional value”, likely due to the various bioactive compounds of the sea grapes and the nutritional benefits for the human nutrition. However, more research is needed to understand the complex interactions between environmental parameters, which vary over regional and temporal scales, and the biochemical composition of the species, in order to potentially increase the production of target-compounds. Additionally, the comparable short shelf-life of the fresh product and the main restriction to the Asian market were identified as bottlenecks for global retail.
In the future, sea grapes could contribute to strengthen the role of green algae in the global seaweed aquaculture sector.
Data availability
The data supporting the conclusions of this manuscript will be made openly available at https://doi.org/10.6084/m9.figshare.21953276 and https://doi.org/10.6084/m9.figshare.21953267.
References
Abreu MH, Pereira R, Yarish C, Buschmann AH, Sousa-Pinto I (2011) IMTA with Gracilaria vermiculophylla: Productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 312:77–87
Ahamad Zakeri H, Abu Bakar L (2013) Copper-, lead- and mercury-induced changes in maximum quantum yield, chlorophyll a content and relative growth of three Malaysian green macroalgae. Malays J Fundam Appl Sci 9:16–21
Alexandre A, Santos R (2020) High nitrogen and phosphorous acquisition by belowground parts of Caulerpa prolifera (Chlorophyta) contribute to the species’ rapid spread in ria formosa lagoon, southern Portugal. J Phycol 56:608–617
Anantpinijwatna A, Nuntamongkol S, Tudkesorn B, Sukchoy O, Deetae P (2018) The kinetic model and temperature effect of Caulerpa lentillifera drying process. In AIP Conference Proceedings. AIP Publishing 2026(1)
Anh NTN, Murungu DK, Khanh LV, Hai TN (2022) Polyculture of sea grape (Caulerpa lentillifera) with different stocking densities of whiteleg shrimp (Litopenaeus vannamei): Effects on water quality, shrimp performance and sea grape proximate composition. Algal Res 67:102845
Anh NTN, Shayo FA, Nevejan N, Van Hoa N (2021) Effects of stocking densities and feeding rates on water quality, feed efficiency, and performance of white leg shrimp Litopenaeus vannamei in an integrated system with sea grape Caulerpa lentillifera. J Appl Phycol 33:3331–3345
Apiratikul R (2017) The simulation of binary component sorption kinetics from the data of single component sorption. Procedia Environ Sci 37:542–548
Apiratikul R (2020) Application of analytical solution of advection-dispersion-reaction model to predict the breakthrough curve and mass transfer zone for the biosorption of heavy metal ion in a fixed bed column. Process Saf Environ Prot 137:58–65
Apiratikul R, Madacha V, Pavasant P (2011) Kinetic and mass transfer analyses of metal biosorption by Caulerpa lentillifera. Desalination 278:303–311
Apiratikul R, Pavasant P (2006) Sorption isotherm model for binary component sorption of copper, cadmium, and lead ions using dried green macroalga, Caulerpa lentillifera. Chem Eng J 119:135–145
Apiratikul R, Pavasant P (2008) Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Bioresour Technol 99:2766–2777
Arimoto A, Nishitsuji K, Higa Y, Arakaki N, Hisato K, Shinzato C, Satoh N, Shoguchi E (2019a) A siphonous macroalgal genome suggests convergent functions of homeobox genes in algae and land plants. DNA Res 26:183–192
Arimoto A, Nishitsuji K, Narisoko H, Shoguchi E, Satoh N (2019b) Differential gene expression in fronds and stolons of the siphonous macroalga, Caulerpa lentillifera. Dev Growth Differ 61:475–484
Arisa I, Zulfikar Z, Muhammadar M, Nurfadillah N, Mellisa S (2020) Study on the addition of Caulerpa lentillifera on growth and survival rate of saline tilapia Oreochromis niloticus, L. IOP Conf Ser Earth Environ Sci 493:012004
Balasubramaniam V, June Chelyn L, Vimala S, Mohd Fairulnizal MN, Brownlee IA, Amin I (2020) Carotenoid composition and antioxidant potential of Eucheuma denticulatum, Sargassum polycystum and Caulerpa lentillifera. Heliyon 6:e04654
Bambaranda BVASM, Sasaki N, Chirapart A, Salin KR, Tsusaka TW (2019a) Optimization of macroalgal density and salinity for nutrient removal by Caulerpa lentillifera from aquaculture effluent. Processes 7:303
Bambaranda BVASM, Tsusaka TW, Chirapart A, Salin KR, Sasaki N (2019b) Capacity of Caulerpa lentillifera in the removal of fish culture effluent in a recirculating aquaculture system. Processes 7:440
Barbier M, Charrier B, Araujo R, Holdt SL, Jacquemin B, Rebours C (2019) PEGASUS—PHYCOMORPH European guidelines for a sustainable aquaculture of seaweeds. Roscoff, France
Benzie JAH, Price IR, Ballment E (1997) Population genetics and taxonomy of Caulerpa (Chlorophyta) from the Great Barrier Reef, Australia. J Phycol 33:491–504
Cahill PL, Hurd CL, Lokman M (2010) Keeping the water clean — Seaweed biofiltration outperforms traditional bacterial biofilms in recirculating aquaculture. Aquaculture 306:153–159
Cai Y, Li G, Zou D, Hu S, Shi X (2021a) Rising nutrient nitrogen reverses the impact of temperature on photosynthesis and respiration of a macroalga Caulerpa lentillifera (Ulvophyceae, Caulerpaceae). J Appl Phycol 33:1115–1123
Cai J, Lovatelli A, Aguilar-Manjarrez J, Cornish L, Dabbadie L, Desrochers A, Diffey S, Garrido Gamarro E, Geehan J, Hurtado A, Lucente D, Mair G, Miao W, Potin P, Przybyla C, Reantaso M, Roubach R, Tauati M, Yuan X (2021a) Seaweeds and microalgae: an overview for unlocking their potential in global aquaculture development. FAO Fisheries and Aquaculture Circular No. 1229, Rome. pp 1–36
Chaiklahan R, Srinorasing T, Chirasuwan N, Tamtin M, Bunnag B (2020) The potential of polysaccharide extracts from Caulerpa lentillifera waste. Int J Biol Macromol 161:1021–1028
Chaitanawisuti N, Santhaweesuk W, Kritsanapuntu S (2011) Performance of the seaweeds Gracilaria salicornia and Caulerpa lentillifera biofilters in a hatchery scale recirculating aquaculture system for juvenile spotted babylons (Babylonia areolata). Aquac Int 19:1139–1150
Chang H-C, Lin Y-K, Lin Y-H, Lin Y-H, Hu W-C, Chiang C-F (2021) Hydrolyzed collagen combined with djulis and green caviar improve skin condition: A randomized, placebo-controlled trial. Curr Res Nutr Food Sci J 9:533–541
Chen X, Sun Y, Liu H, Liu S, Qin Y, Li P (2019) Advances in cultivation, wastewater treatment application, bioactive components of Caulerpa lentillifera and their biotechnological applications. PeerJ 7:e6118
Chopin T, Tacon AGJJ (2021) Importance of seaweeds and extractive species in global aquaculture production. Rev Fish Sci Aquac 29:139–148
Chung I-K, Kang Y-H, Yarish C, Kraemer GP, Lee JA (2002) Application of seaweed cultivation to the bioremediation of nutrient-rich effluent. Algae 17:187–194
Comerford B, Paul N, Marshall D (2021) Effects of light variation in algal cultures: A systematic map of temporal scales. J Appl Phycol 33:3483–3496
Darmawan M, Fajarningsih ND, Sihono IHE (2020) Caulerpa: Ecology, nutraceutical and pharmaceutical potential. In: Nathani NM, Mootapally C, Gadhvi IR, Maitreya B, Joshi CG (eds) Marine Niche: Applications in pharmaceutical sciences. Springer, Singapore, pp 299–318
Daud D, Zainal AN, Nordin MN, Tawang A, Ismail A (2020) Chelation activity and protective effect of Caulerpa lentillifera aqueous extract against lead acetate-induced toxicity in Sprague Dawley rats. J Appl Pharm Sci 10:145–148
Debbarma J, Madhusudana Rao B, Murthy LN, Mathew S, Venkateshwarlu G, Ravishankar CN (2016) Nutritional profiling of the edible seaweeds Gracilaria edulis, Ulva lactuca and Sargassum sp. Indian J Fish 63:81–87
de Boer J, Hoogland CT, Boersema JJ (2007) Towards more sustainable food choices: Value priorities and motivational orientations. Food Qual Prefer 18:985–996
de Gaillande C, Payri C, Remoissenet G, Zubia M (2017) Caulerpa consumption, nutritional value and farming in the Indo-Pacific region. J Appl Phycol 29:2249–2266
Dobson GT, Duy NDQ, Paul NA, Southgate PC (2020) Assessing potential for integrating sea grape (Caulerpa lentillifera) culture with sandfish (Holothuria scabra) and Babylon snail (Babylonia areolata) co-culture. Aquaculture 522:735153
Dring MJ (2005) Stress resistance and disease resistance in seaweeds: The role of reactive oxygen metabolism. Adv Bot Res 43:175–207
Dumilag RV (2019) Edible seaweeds sold in the local public markets in Tawi-Tawi, Philippines. Philipp J Sci 148:803–811
Estrada JL, Arboleda MDM, Dionisio-Sese ML (2021) Current status of sea grapes (Caulerpa spp.) farming and wild harvesting in the Philippines. J Appl Phycol 33:3215–3223
Fajriah S, Handayani S, Sinurat E, Megawati M, Darmawan A, Hariyanti H, Dewi RT, Wira Septama A (2020) In vitro immunomodulatory effect from edible green seaweed of Caulerpa lentillifera extracts on nitric oxide production and phagocytosis activity of RAW 264.7 murine macrophage cells. J Young Pharm 12:334–337
Fakhrulddin IM, Harah ZM, Shiamala RD, Azrie AM (2021) Effect of salinity, light and fertilizer on Caulerpa lentillifera under culture conditions. AIP Conf Proc 2347:020074
FAO (2020) The State of World Fisheries and Aquaculture 2020. FAO, Rome
Gao D, Huang C, Yao J, Yao J, Li Y, Tan W, Sun Z (2018) Characterization of the whole chloroplast genome Caulerpa lentillifera J. Agardh (Bryopsidales, Chlorophyta). Mitochondrial DNA B 3:1198–1199
Gao D, Sun Z, Huang C, Yao J, Wang Y, Tan W, Chen F (2020) First record of Caulerpa lentillifera J. Agardh (Bryopsidales, Chlorophyta) from China. Mar Biol Res 16:44–49
Guo H, Yao J, Sun Z, Duan D (2015a) Effects of salinity and nutrients on the growth and chlorophyll fluorescence of Caulerpa lentillifera. Chin J Oceanol Limnol 33:410–418
Guo H, Yao J, Sun Z, Duan D (2015b) Effect of temperature, irradiance on the growth of the green alga Caulerpa lentillifera (Bryopsidophyceae, Chlorophyta). J Appl Phycol 27:879–885
Honwichit O, Ruengsaengrob P, Buathongjan C, Charoensiddhi S (2022) Influence of extraction methods on the chemical composition and antioxidant activity of polysaccharide extracts from discarded sea grape (Caulerpa lentillifera). J Fish Environ 46:169–179
Ilias NN, Jamal P, Jaswir I, Sulaiman S, Zainudin Z, Azmi AS (2015) Potentiality of selected seaweed for the production of nutritious fish feed using solid state fermentation. J Eng Sci Technol 10:30–40
Ismail MF, Ramaiya SD, Zakaria MH, Mohd Ikhsan NF, Awang MA (2020) Mineral content and phytochemical properties of selected Caulerpa species from Malaysia. Malays J Sci 39:115–131
Jia X, Liu T, Wang X, Tang X, Jin Y (2019) The complete mitogenome of Caulerpa lentillifera and its phylogenetic analysis. Mitochondrial DNA B 4:3169–3170
Kang L-K, Huang Y-J, Lim W-T, Hsu P-H, Hwang P-A (2020) Growth, pigment content, antioxidant activity, and phytoene desaturase gene expression in Caulerpa lentillifera grown under different combinations of blue and red light-emitting diodes. J Appl Phycol 32:1971–1982
Karadag A, Ozcelik B, Saner S (2009) Review of methods to determine antioxidant capacities. Food Anal Meth 2:41–60
Kazi MA, Reddy CRKK, Jha B (2013) Molecular phylogeny and barcoding of Caulerpa (Bryopsidales) based on the tufA, rbcL, 18S rDNA and ITS rDNA genes. PLoS One 8:e82438
Khairuddin K, Sudirman S, Huang L, Kong Z-L (2020) Caulerpa lentillifera polysaccharides-rich extract reduces oxidative stress and proinflammatory cytokines levels associated with male reproductive functions in diabetic mice. Appl Sci 10:8768
Kopprio GA, Luyen ND, Cuong LH, Duc TM, Fricke A, Kunzmann A, Huong LM, Gärdes A (2021) Insights into the bacterial community composition of farmed Caulerpa lentillifera: A comparison between contrasting health states. Microbiologyopen 10:e1253
Kudaka J, Horii T, Tamanaha K, Itokazu K, Nakamura M, Taira K, Nidaira M, Okano S, Kitahara A (2010) Evaluation of the petrifilm aerobic count plate for enumeration of aerobic marine bacteria from seawater and Caulerpa lentillifera. J Food Prot 73:1529–1532
Lähteenmäki-Uutela A, Rahikainen M, Lonkila A, Yang B (2021) Alternative proteins and EU food law. Food Control 130:108336
Largo DB, Diola AG, Marababol MS (2016) Development of an integrated multi-trophic aquaculture (IMTA) system for tropical marine species in southern Cebu, Central Philippines. Aquac Rep 3:67–76
Leal MC, Munro MHG, Blunt JW, Puga J, Jesus B, Calado R, Rosa R, Madeira C (2013) Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat Prod Rep 30:1380–1390
Li R, Zhang T, Zhong H, Song W, Zhou Y, Yin X (2021) Bioadsorbents from algae residues for heavy metal ions adsorption: chemical modification, adsorption behaviour and mechanism. Environ Technol 42:3132–3143
Liang Z, Liu F, Wang W, Zhang P, Sun X, Wang F, Kell H (2019) High-throughput sequencing revealed differences of microbial community structure and diversity between healthy and diseased Caulerpa lentillifera. BMC Microbiol 19:255
Liang Z, Liu F, Wang W, Zhang P, Yuan Y, Yao H, Sun X, Wang F (2021) A reasonable strategy for Caulerpa lentillifera J. Agardh (Bryopsidales, Chlorophyta) transportation based on the biochemical and photophysiological responses to dehydration stress. Algal Res 56:102304
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol 62:e1–e34
Liu H, Wang F, Wang Q, Dong S, Tian X (2016) A comparative study of the nutrient uptake and growth capacities of seaweeds Caulerpa lentillifera and Gracilaria lichenoides. J Appl Phycol 28:3083–3089
Long H, Gu X, Zhou N, Zhu Z, Wang C, Liu X, Zhao M (2020a) Physicochemical characterization and bile acid-binding capacity of water-extract polysaccharides fractionated by stepwise ethanol precipitation from Caulerpa lentillifera. Int J Biol Macromol 150:654–661
Long H, Gu X, Zhu Z, Wang C, Xia X, Zhou N, Liu X, Zhao M (2020b) Effects of bottom sediment on the accumulation of nutrients in the edible green seaweed Caulerpa lentillifera (sea grapes). J Appl Phycol 32:705–716
Lu J, Zhang C, Wu J (2021) Removal of steroid hormones from mariculture system using seaweed Caulerpa lentillifera. Front Environ Sci Eng 16:15
Lusk JL (2017) Consumer research with big data: Applications from the food demand survey (FooDS). Am J Agric Econ 99:303–320
Ly KV, Murungu DK, Nguyen DP, Nguyen NAT (2021) Effects of different densities of sea grape Caulerpa lentillifera on water quality, growth and survival of the whiteleg shrimp Litopenaeus vannamei in polyculture system. Fishes 6:19
Magnusson M, Mata L, Wang N, Zhao J, de Nys R, Paul NA (2015) Manipulating antioxidant content in macroalgae in intensive land-based cultivation systems for functional food applications. Algal Res 8:153–160
Manoppo JIC, Nurkolis F, Pramono A, Ardiaria M, Qhabibi FR, Yusuf VM, Amar N, Rico M, Karim A, Subali AD, Natanael H, Rompies R, Halim RF, Sam A, Bolang L, Joey G, Novianto CA, Permatasari HK (2022) Amelioration of obesity-related metabolic disorders via supplementation of amelioration of obesity-related metabolic disorders via supplementation of Caulerpa lentillifera in rats fed with a high-fat and high-cholesterol. Front Nutr 9:1010867
Mantri VA (2004) Rediscovery of Caulerpa lentillifera: A potential food alga from Samiani Island, West coast of India. Curr Sci 87:1321–1322
Margono AJT, Nurhudah M (2021) Effectiveness of seaweed (Caulerpa lentillifera) as biofilter in Vanamei shrimp (Litopenaeus vannamei) culture. AACL Bioflux 14:1734–1746
Marinho-Soriano E, Nunes SO, Carneiro MAA, Pereira DC (2009) Nutrients’ removal from aquaculture wastewater using the macroalgae Gracilaria birdiae. Biomass Bioenergy 33:327–331
Marques R, Cruz S, Calado R, Lillebø A, Abreu H, Pereira R, Pitarma B, da Silva JM, Cartaxana P (2021) Effects of photoperiod and light spectra on growth and pigment composition of the green macroalga Codium tomentosum. J Appl Phycol 33:471–480
Marungrueng K, Pavasant P (2007) High performance biosorbent (Caulerpa lentillifera) for basic dye removal. Bioresour Technol 98:1567–1572
Marungrueng K, Pavasant P (2006) Removal of basic dye (Astrazon Blue FGRL) using macroalga Caulerpa lentillifera. J Environ Manage 78:268–274
Mary A, Mary V, Lorella A, Jonathan RM (2009) Rediscovery of naturally occurring seagrape Caulerpa lentillifera from the Gulf of Mannar and its mariculture. Curr Sci 97:1418–1420
Matanjun P, Mohamed S, Mustapha NM, Muhammad K, Ming CH (2008) Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J Appl Phycol 20:367–373
Matanjun P, Mohamed S, Mustapha NM, Muhammad K (2009) Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J Appl Phycol 21:75–80
Minh NP, Nhi TTY, Tuyen LK, Phi TH, Khoa DT, Thuan TQ (2019) Technical factors affecting seagrape (Caulerpa lentillifera) production by cultivation and Its stability by post-harvest treatment. J Pharm Sci Res 11:783–786
Miyamura S, Hori T (1991) DNA is present in the pyrenoid core of the siphonous green algae of the genus Caulerpa and yellow-green algae of the genus Pseudodichotomosiphon. Protoplasma 161:192–196
Miyamura S, Hori T (1995) Further confirmation of the presence of DNA in the pyrenoid core of the siphonous green algal genus Caulerpa (Caulerpales, Ulvophyceae, Chlorophyta). Phycol Res 43:101–104
Moreira A, Cruz S, Marques R, Cartaxana P (2021) The underexplored potential of green macroalgae in aquaculture. Rev Aquacult 1:5–26
Mouritsen OG, Rhatigan P, Pérez-Lloréns JL (2019) The rise of seaweed gastronomy: phycogastronomy. Bot Mar 62:195–209
Nagappan T, Vairappan CS (2014) Nutritional and bioactive properties of three edible species of green algae, genus Caulerpa (Caulerpaceae). J Appl Phycol 26:1019–1027
Nakamura M, Kumagai NH, Tamaoki M, Arita K, Ishii Y, Nakajima N, Yabe T (2020) Photosynthesis and growth of Ulva ohnoi and Ulva pertusa (Ulvophyceae) under high light and high temperature conditions, and implications for green tide in Japan. Phycol Res 68:152–160
Neori A, Chopin T, Troell M, Buschmann AH, Kraemer GP, Halling C, Shpigel M, Yarish C (2004) Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 231:361–391
Nguyen VT, Ueng J-PP, Tsai G-JJ (2011) Proximate composition, total phenolic content, and antioxidant activity of seagrape (Caulerpa lentillifera). J Food Sci 76:C950–C958
Nufus C, Abdullah A, Nurjanah, (2019) Characteristics of green seaweed salt as alternative salt for hypertensive patients. IOP Conf Ser Earth Environ Sci 278:012050
Omont A, Peña-Rodríguez A, Terauchi S, Matsui A, Magallón Barajas F, Torres-Ochoa E, Endo M (2022) Growth performance and mineral composition of the white shrimp Penaeus vannamei and the sea grape Caulerpa lentillifera in a co-culture system. Aquac Res 53:6487–6499
Ong MY, Abdul Latif NIS, Leong HY, Salman B, Show PL, Nomanbhay S (2019) Characterization and analysis of Malaysian macroalgae biomass as potential feedstock for bio-oil production. Energies 12:3509
Pang M, Huang Z, Lv L, Li X, Jin G (2022) Seasonal succession of bacterial communities in cultured Caulerpa lentillifera detected by high-throughput sequencing. Open Life Sci 17:10–21
Paul NA, de Nys R (2008) Promise and pitfalls of locally abundant seaweeds as biofilters for integrated aquaculture. Aquaculture 281:49–55
Paul NA, Neveux N, Magnusson M, de Nys R (2014) Comparative production and nutritional value of “sea grapes” - the tropical green seaweeds Caulerpa lentillifera and C. racemosa. J Appl Phycol 26:1833–1844
Pavasant P, Apiratikul R, Sungkhum V, Suthiparinyanont P, Wattanachira S, Marhaba TF (2006) Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour Technol 97:2321–2329
Pimol P, Khanidtha M, Prasert P (2008) Influence of particle size and salinity on adsorption of basic dyes by agricultural waste: dried seagrape (Caulerpa lentillifera). J Environ Sci 20:760–768
Polo LK, Chow F (2022) Variation of antioxidant capacity and antiviral activity of the brown seaweed Sargassum filipendula (Fucales, Ochrophyta) under UV radiation treatments. Appl Phycol 3:260–273
Putra DF, Rahmawati M, Abidin MZ, Ramlan R (2019) Dietary administration of sea grape powder (Caulerpa lentillifera) effects on growth and survival rate of black tiger shrimp (Penaeus monodon). IOP Conf Ser Earth Environ Sci 348:012100
Rabia MDS (2016) Cultivation of Caulerpa lentillifera using tray and sowing methods in brackishwater pond. Environ Sci 4:23–29
Ratana-arporn P, Chirapart A (2006) Nutritional evaluation of tropical green seaweeds Caulerpa lentillifera and Ulva reticulata. Kasetsart J - Nat Sci 40:75–83
Roleda MY, Hurd CL (2019) Seaweed nutrient physiology: application of concepts to aquaculture and bioremediation. Phycologia 58:552–562
Saito H, Xue C, Yamashiro R, Moromizato S, Itabashi Y (2010) High polyunsatturated fatty acid levels in two subtropical macroalgae, Cladosiphon okamuranus and Caulerpa lentillifera. J Phycol 46:665–673
Salleh A, Wakid SA (2008) Nutritional composition of macroalgae in Tanjung Tuan, Port Dickson, Malaysia. Malays J Sci 27:19–26
Setthamongkol P, Tunkijjanukij S, Satapornvanit K, Salaenoi J (2015) Growth and nutrients analysis in marine macroalgae. Kasetsart J - Nat Sci 49:211–218
Shevchenko NM, Burtseva YV, Zvyagintseva TN, Makar’eva TN, Sergeeva OS, Zakharenko AM, Isakov VV, Thi Linh N, Xuan Hoa N, Minh Ly B, Van Huyen P (2009) Polysaccharides and sterols from green algae Caulerpa lentillifera and C. sertularioides. Chem Nat Compd 45:1–5
Sommer J, Kunzmann A, Stuthmann LE, Springer K (2022) The antioxidative potential of sea grapes (Caulerpa lentillifera, Chlorophyta) can be triggered by light to reach comparable values of pomegranate and other highly nutritious fruits. Plant Physiol Rep 27:186–191
Srinorasing T, Chirasuwan N, Bunnag B, Chaiklahan R (2021) Lipid extracts from Caulerpa lentillifera waste: An alternative product in a circular economy. Sustainability 13:4491
Stengel DB, Connan S, Popper ZA (2011) Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol Adv 29:483–501
Stuthmann LE, Achuthan R, Pribbernow M, Springer K, Kunzmann A (2022) Improving the nutritional value of edible Caulerpa lentillifera (Chlorophyta ) using high light intensities. A realistic tool for sea grape farmers. Algal Res 66:102785
Stuthmann LE, Springer K, Kunzmann A (2020) Cultured and packed sea grapes (Caulerpa lentillifera): effect of different irradiances on photosynthesis. J Appl Phycol 33:1125–1136
Sulaimana AS, Chang C-KK, Hou C-YY, Yudhistra B, Punthi F, Lung C-T, Cheng K-C, Satoso S-P, Hsieh C-W (2021) Effect of oxidative stress on physicochemical quality of Taiwanese seagrape (Caulerpa lentillifera) with the application of alternating current electric field (ACEF) during post-harvest storage. Processes 9:1011
Syakilla N, George R, Chye FY, Pindi W, Mantihal S, Wahab NA, Fadzwi FM, Gu PH, Matanjun P (2022) A review on nutrients, phytochemicals, and health benefits of green seaweed, Caulerpa lentillifera. Foods 11:2832
Syamsuddin R, Azis HY, Badraeni R (2019) Comparative study on the growth, carotenoid, fibre and mineral content of the seaweed Caulerpa lentillifera cultivated indoors and in the sea. IOP Conf Ser Earth Environ Sci 370:012019
Tanaka Y, Ashaari A, Mohamad FS, Lamit N (2020) Bioremediation potential of tropical seaweeds in aquaculture: Low-salinity tolerance, phosphorus content, and production of UV-absorbing compounds. Aquaculture 518:734853
Tanduyan SN, Gonzaga RB, Bensig VD (2013) Off bottom culture of Caulerpa lentillifera in three different water levels in the marine waters of San Francisco, Cebu, Philippines. Galaxea, J Coral Reef Stud 15:123–132
Terada R, Nakazaki Y, Borlongan IA, Endo H, Nishihara GN (2018) Desiccation effect on the PSII photochemical efficiency of cultivated Japanese Caulerpa lentillifera under the shipping package environment. J Appl Phycol 30:2533–2538
Terada R, Takaesu M, Borlongan IA, Nishihara GN (2021) The photosynthetic performance of a cultivated Japanese green alga Caulerpa lentillifera in response to three different stressors, temperature, irradiance, and desiccation. J Appl Phycol 33:2547–2559
Terriente-palacios C, Castellari M (2022) Levels of taurine, hypotaurine and homotaurine, and amino acids profiles in selected commercial seaweeds, microalgae, and algae-enriched food products. Food Chem 368:130770
Thu NTH, Anh HTL, Hien HTM, Ha NC, Tam LT, Khoi TX, Duc TM, Hong DD (2018) Preparation and evaluation of cream mask from Vietnamese seaweeds. J Cosmet Sci 69:447–462
Tolentino PDH, Sorio JC, Tolentino DHP, Sorio JC (2021) Quality changes in sea grape, Caulerpa lentillifera at different brine concentrations. J Fish 9:91204
Trono GC, Largo DB (2019) The seaweed resources of the Philippines. Bot Mar 62:483–498
Trono CG, Toma T (1993) Cultivation of the green alga Caulerpa lentillifera. In: Ohno M, Critchley AT (eds) Seaweed cultivation and Marine Ranching. Kanagawa International Fisheries Training Center, JICA, Yokosuka, pp 17–24
Wendin K, Undeland I (2020) Seaweed as food – Attitudes and preferences among Swedish consumers. A pilot study. Int J Gastron Food Sci 22:100265
Wichachucherd B, Pannak S, Saengthong C, Koodkaew I, Rodcharoen E (2019) Correlation between growth, phenolic content and antioxidant activity in the edible seaweed Caulerpa lentillifera in open pond culture system. J Fish Environ 43:66–75
Wuttilerts T, Chulalaksananukul S, Peerapongpipat P, Suksommanat P (2019) Evaluation of biodiesel production using oil feedstock from contaminated macro algae in shrimp farming. Appl Sci Eng Prog 12:179–185
Yap WG (1999) Rural Aquaculture in the Philippines. FAO, Regional Office for Asia and the Pacific, Bankok. RAP Publ 82:1–82
Yoojam S, Ontawong A, Lailerd N, Mengamphan K, Amornlerdpison D (2021) The enhancing immune response and anti-inflammatory effects of Caulerpa lentillifera extract in RAW 264.7 cells. Molecules 26:5734
You Y, Song H, Wang L, Peng H, Sun Y, Ai C, Wen C, Zhu B, Song S (2022) Structural characterization and SARS-CoV-2 inhibitory activity of a sulfated polysaccharide from Caulerpa lentillifera. Carbohydr Polym 280:119006
Zhang M, Ma Y, Che X, Huang Z, Chen P, Xia G, Zhao M (2020) Comparative analysis of nutrient composition of Caulerpa lentillifera from different egions. J Ocean Univ China 19:439–445
Zheng F, Liu H, Jiang M, Xu Z, Wang Z, Wang C, Du F, Shen Z, Wang B (2018) The complete mitochondrial genome of the Caulerpa lentillifera (Ulvophyceae, Chlorophyta): Sequence, genome content, organization structure and phylogenetic consideration. Gene 673:225–238
Zubia M, Draisma SGA, Morrissey KL, Varela-Álvarez E, De Clerck O (2020) Concise review of the genus Caulerpa J.V. Lamouroux. J Appl Phycol 32:23–39
Funding
Open Access funding enabled and organized by Projekt DEAL. Funding has been received from the Leibniz Centre for Tropical Marine Research inhouse-funds.
Author information
Authors and Affiliations
Contributions
Lara E. Stuthmann: Conceptualization (lead), Investigation (equal), Visualization (equal), Methodology (equal), Investigation (equal), Writing – Original Draft Preparation; Beatrice Brix da Costa: Conceptualization (supporting), Investigation (equal), Visualization (equal), Methodology (equal), Investigation (equal), Writing – Original Draft Preparation (equal); Karin Springer: Writing – Review & Editing (equal), Supervision (equal), Funding Acquisition (equal); Andreas Kunzmann: Writing – Review & Editing (equal), Supervision (equal), Funding Acquisition (equal).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest disclosure
The authors declare no competing conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lara Elisabeth Stuthmann, Beatrice Brix da Costa joined first-authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stuthmann, L.E., Brix da Costa, B., Springer, K. et al. Sea grapes (Caulerpa lentillifera J. Agardh, Chlorophyta) for human use: Structured review on recent research in cultivation, nutritional value, and post-harvest management. J Appl Phycol 35, 2957–2983 (2023). https://doi.org/10.1007/s10811-023-03031-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03031-x