Abstract
Purpose of Review
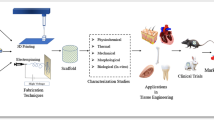
Consistently, a huge number of surgeries are performed to supplant tissue which is damaged through infection or injury. The emerging field of tissue engineering aims to recover injured tissues by consolidating cells from the host body or donor’s body with exceptionally porous scaffold biomaterials that can act as a template for tissue recovery, to control the development of new tissue. Signals, cells, and scaffolds are triad components of tissue engineering that combine to produce functional tissue and organs.
Recent Findings
Pubmed and Google Scholar are the search engines used to sort out relevant papers on nanoscaffolds and their application in tissue engineering and regenerative medicine. Nanoscaffolding is known to be a clinical cycle used to regrow bone and tissue, including appendages and organs; likewise, it has been utilized to regrow the skin, but it has not been utilized yet for the development of complex organs like the heart etc. Different synthesis methods are being employed to engineer the scaffolds, such as electrospinning, layer-by-layer assembly, 3D printing, particle leaching etc. Natural bioscaffolds have also been used for growing cells and regenerative biology.
Summary
This article portrays the functional scaffolds used in different kinds of tissue engineering such as bone, liver, cartilage, vascular tissue, skin and cardiac tissue, etc., and an overview of various types of materials used in scaffolding for the tissue engineering applications and future aspects is discussed.



Similar content being viewed by others
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Abbreviations
- TE:
-
Tissue engineering
- ECM:
-
Extracellular matrix
- SIS:
-
Small intestine submucosa
- GMP:
-
Good manufacturing practice
- KCl:
-
Potassium chloride
- NaCl:
-
Sodium chloride
- PCL:
-
Polycaprolactone
- PEO:
-
Polyethylene oxide
- PBS:
-
Polybutylene succinate
- CNC:
-
Cellulose nanocrystals
- Wt:
-
Weight
- h:
-
Hour
- NPs:
-
Nanoparticles
- PNIPAm:
-
Poly(N-isopropylacrylamide)
- AuNP:
-
Gold nanoparticle
- TiO2 :
-
Titanium dioxide
- Ti:
-
Titanium
- PLGA:
-
Poly(lactide-co-glycolic) acid
- PLA:
-
Polylactic acid
- AgNPs:
-
Silver nanoparticles
- IL-6:
-
Interleukin 6
- IL-10:
-
Interleukin 10
- MMPs:
-
Matrix metalloproteinase
- PMNs:
-
Polymorphonuclear leukocytes
- BMP-2 peptide:
-
Bone morphogenetic protein 2
- HCAEC:
-
Human coronary artery endothelial cells
- PHAs:
-
Polyhydroxyalkanoates (PHAs)
- CNT’s:
-
Carbon nanotubes
- iPSC:
-
Induced pluripotent stem cells
- QOS:
-
Quaternary ammonium silane
- CEF:
-
Cephalexin monohydrate
- HNT:
-
Halloysite nanotubes
- PVA:
-
Poly(vinyl alcohol)
- nHAp:
-
Nano-hydroxyapatite
- PG-NFs:
-
Poly(vinyl alcohol)-gellan gum nanofiber
- SEM:
-
Scanning electron microscopy
- FTIR:
-
Fourier transformation infrared spectroscopy
- hDPSCs:
-
Human dental pulp–derived stem cells
- CMC:
-
Carboxymethyl chitosan
- wjhMSC-MT:
-
Wharton’s jelly–derived mesenchymal stem cell microtissue
- ALP:
-
Alkaline phosphatase
- OC:
-
Csteocalcin
- CNF:
-
Cellulose nanofibrils
- RGO:
-
Reduced graphene oxide
- BM-MSC:
-
Bone marrow–mesenchymal stem cells
- DNA:
-
Deoxyribose nucleic acid
- HFB4 cells:
-
Human fibroblast cell lines
- AT-MSCs:
-
Adipose tissue–derived mesenchymal stem cells
- hFOB cells:
-
Human osteoblastic cells
- MC3T3 cell lines:
-
Osteoblast precursor cells
- MG-63 cell lines:
-
Human osteosarcoma cells
- WI-38 cells:
-
Human lung fibroblast cells
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mhanna R, Hasan A. Introduction to tissue engineering, Tissue engineering for artificial organs: regenerative medicine, smart diagnostics and personalized medicine. Hasan A, editor. Wiley-VCH Verlag GmbH & Co. KGaA. 2017. pp.1–34. https://doi.org/10.1002/9783527689934.ch1.
Pandey AR, Singh US, Momin M, Bhavsar C. Chitosan: application in tissue engineering and skin grafting. J Polym Res. 2017;24(8):1–22. https://doi.org/10.1007/s10965-017-1286-4.
Hasan A, Morshed M, Memic A, Hassan S, Webster TJ, Marei HE. Nanoparticles in tissue engineering: applications, challenges and prospects. Int J Nanomedicine. 2018;13:5637–55. https://doi.org/10.2147/IJN.S153758.
Pina S, Ribeiro VP, Marques CF, Maia FR, Silva TH, Reis RL, Oliveira JM. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials. 2019;12(11):1824. https://doi.org/10.3390/ma12111824.
Girigoswami K, Akhtar N. Nanobiosensors and fluorescence based biosensors: an overview. Int Nano Dimens. 2019;10(1):1–17. http://www.ijnd.ir/article_660965.html
Metkar SK, Girigoswami K. Diagnostic biosensors in medicine- a review. Biocatal Agric Biotechnol. 2019;17:271–83. https://doi.org/10.1016/j.bcab.2018.11.029.
Thendral V, Dharshni T, Ramalakshmi M, Girigoswami A, Girigoswami K. Cerium oxide nanocluster based nanobiosensor for ROS detection. Biocatal Agric Biotechnol. 2019;19: 101124. https://doi.org/10.1016/j.bcab.2019.101124.
Akhtar N, Metkar SK, Girigoswami A, Girigoswami K. ZnO nanoflower based sensitive nano-biosensor for amyloid detection. Mater Sci Eng C. 2017;78:960–8. https://doi.org/10.1016/j.msec.2017.04.118.
Girigoswami K, Girigoswami A. A review on role of nanosensors in detecting cellular miRNA expression in colorectal cancer. Endocr Metab Immune Disord Drug Targets. 2021;21(1):12–26. https://doi.org/10.2174/1871530320666200515115723.
Deepika R, Girigoswami K, Murugesan R, Girigoswami A. Influence of divalent cation on morphology and drug delivery efficiency of mixed polymer nanoparticles. Curr drug deliv. 2018;15(5):652–7. https://doi.org/10.2174/1567201814666170825160617.
Girigoswami A, Yassine W, Sharmiladevi P, Haribabu V, Girigoswami K. Camouflaged nanosilver with excitation wavelength dependent high quantum yield for targeted theranostic. Sci reports. 2018;8(1):1–7. https://doi.org/10.1038/s41598-018-34843-4.
Sharmiladevi P, Akhtar N, Haribabu V, Girigoswami K, Chattopadhyay S, Girigoswami A. Excitation wavelength independent carbon decorated ferrite nanodots for multimodal diagnosis and stimuli responsive therapy. ACS Appl Biomater. 2019;2(4):1634–42. https://doi.org/10.1021/acsabm.9b00039.
Ghosh S, Girigoswami K, Girigoswami A. Membrane-encapsulated camouflaged nanomedicines in drug delivery. Nanomedicine. 2019;14(15):2067–82. https://doi.org/10.2217/nnm-2019-0155.
Sharmiladevi P, Breghatha M, Dhanavardhini K, Priya R, Girigoswami K, Girigoswami A. Efficient wormlike micelles for the controlled delivery of anticancer drugs. Nanosci Nanotechnol Asia. 2021;11(3):350–6. https://doi.org/10.2174/2210681210999200728115601.
Sharmiladevi P, Girigoswami K, Haribabu V, Girigoswami A. Nano-enabled theranostics for cancer. Materials. Advances. 2021;2:2876–91. https://doi.org/10.1039/D1MA00069A.
Haribabu V, Girigoswami K, Sharmiladevi P, Girigoswami A. Water-nanomaterials interaction to escalate twin-mode magnetic resonance imaging. ACS Biomater Sci Eng. 2020;6(8):4377–89. https://doi.org/10.1021/acsbiomaterials.0c00409.
Haribabu V, Sharmiladevi P, Akhtar N, Farook AS, Girigoswami K, Girigoswami A. Label free ultrasmall fluoromagnetic ferrite-clusters for targeted cancer imaging and drug delivery. Curr Drug Deliv. 2019;16(3):233–41. https://doi.org/10.2174/1567201816666181119112410.
Sharmiladevi P, Haribabu V, Girigoswami K, Farook AS, Girigoswami A. Effect of mesoporous nano water reservoir on MR relaxivity. Sci reports. 2017;7(1):1–7. https://doi.org/10.1038/s41598-017-11710-2.
Pallavi P, Sharmiladevi P, Haribabu V, Girigoswami K, Girigoswami A. A nano approach to formulate photosensitizers for photodynamic therapy. Curr Nanosci. 2022;18:1. https://doi.org/10.2174/1573413718666211222162041.
Agraharam G, Girigoswami A, Girigoswami K. Nanoencapsulated myricetin to improve antioxidant activity and bioavailability: a study on zebrafish embryos. Chemistry. 2022;4:1–17. https://doi.org/10.3390/chemistry4010001.
De S, Gopikrishna A, Keerthana V, Girigoswami A, Girigoswami K. An overview of nanoformulated nutraceuticals and their therapeutic approaches. Curr Nutr Food Sci. 2021;17(4):392–407. https://doi.org/10.2174/1573401316999200901120458.
•Girigoswami K, Srinivasan N, Girigoswami A. Fate of stem cells grown on the extracellular matrix isolated from cancer cells and their possible applications in tissue engineering. Curr Sci. 2021a;120(10): 1616–1622. This paper discusses about the stem cell fate when grown above the extracellular matrix isolated from different types of cancer cells.
•Girigoswami K, Saini D, Girigoswami A. Extracellular matrix remodeling and development of cancer. Stem Cell Rev. 2021b;17(3):739–747. https://doi.org/10.1007/s12015-020-10070-1. This paper discusses about the extraceelular matrix remodeling and its role in cancer.
Zhao P, Gu H, Mi H, Rao C, Fu J, Turng LS. Fabrication of scaffolds in tissue engineering: a review. Front Mech Eng. 2018;13(1):107–19. https://doi.org/10.1007/s11465-018-0496-8.
Wahid F, Khan T, Hussain Z, Ullah H. 30 - Nanocomposite scaffolds for tissue engineering; properties, preparation and applications. Applications of Nanocomposite Materials in Drug Deliver. 2018;701–735. https://doi.org/10.1016/B978-0-12-813741-3.00031-5
Reignier J, Huneault MA. Preparation of interconnected poly (ε-caprolactone) porous scaffolds by a combination of polymer and salt particulate leaching. Polymer. 2006;47(13):4703–17. https://doi.org/10.1016/j.polymer.2006.04.029.
Akbarzadeh R, Yousefi AM. Effects of processing parameters in thermally induced phase separation technique on porous architecture of scaffolds for bone tissue engineering. J Biomed Mater Res Part B Appl Biomater. 2014;102(6):1304–15. https://doi.org/10.1002/jbm.b.33101.
• Greiner A, Wendorff JH. Electrospinning: a fascinating method for the preparation of ultrathin fibers. Angew Chem Int Ed. 2007;46(30):5670–703. https://doi.org/10.1002/anie.200604646. (This article explained basic concepts of electrospinning method.)
Jun I, Han HS, Edwards JR, Jeon H. Electrospun fibrous scaffolds for tissue engineering: viewpoints on architecture and fabrication. Int J Mol Sci. 2018;19(3):745. https://doi.org/10.3390/ijms19030745.
Huang A, Peng X, Geng L, Zhang L, Huang K, Chen B, Gu Z, Kuang T. Electrospun poly (butylene succinate)/cellulose nanocrystals bio-nanocomposite scaffolds for tissue engineering: Preparation, characterization and in vitro evaluation. Polym Test. 2018;71:101–9. https://doi.org/10.1016/j.polymertesting.2018.08.027.
Jin G, He R, Sha B, Li W, Qing H, Teng R, Xu F. Electrospun three-dimensional aligned nanofibrous scaffolds for tissue engineering. Mater Sci Eng C Mater Biol Appl. 2018;92:995–1005. https://doi.org/10.1016/j.msec.2018.06.065.
Janmohammadi M, Nourbakhsh MS. Electrospun polycaprolactone scaffolds for tissue engineering: a review. Int J Polym Mater Polym Biomater. 2019;68(9):527–39. https://doi.org/10.1080/00914037.2018.1466139.
Nazeer MA, Yilgor E, Yilgor I. Electrospun polycaprolactone/silk fibroin nanofibrous bioactive scaffolds for tissue engineering applications. Polymer. 2019;168:86–94. https://doi.org/10.1016/j.polymer.2019.02.023.
Teixeira MA, Amorim MTP, Felgueiras HP. Poly(vinyl alcohol)-based nanofibrous electrospun scaffolds for tissue engineering applications. Polymers (Basel). 2019;12(1):7. https://doi.org/10.3390/polym12010007.
Pugliese R, Maleki M, Zuckermann RN, Gelain F. Self-assembling peptides cross-linked with genipin: resilient hydrogels and self-standing electrospun scaffolds for tissue engineering applications. Biomater sci. 2019;7(1):76–91. https://doi.org/10.1039/C8BM00825F
Tan GZ, Zhou Y. Electrospinning of biomimetic fibrous scaffolds for tissue engineering: a review. Int J Polym Mater Polym Biomater. 2020;69(15):947–60. https://doi.org/10.1080/00914037.2019.1636248.
••Parham S, Kharazi AZ, Bakhsheshi-Rad HR, Ghayour H, Ismail AF, Nur H, Berto F. Electrospun nano-fibers for biomedical and tissue engineering applications: a comprehensive review. Materials. 2020;13(9):2153. https://doi.org/10.3390/ma13092153. This article reviewed multiple parameters for different types of materials/polymers for the formation of scaffolds by using electrospinning method.
Rahmati M, Mills DK, Urbanska AM, Saeb MR, Venugopal JR, Ramakrishna S, Mozafari M. Electrospinning for tissue engineering applications. Prog Mater Sci. 2021;117: 100721. https://doi.org/10.1016/j.pmatsci.2020.100721.
Zhang S, Xing M, Li B. Biomimetic layer-by-layer self-assembly of nanofilms, nanocoatings, and 3D scaffolds for tissue engineering. Int J Mol Sci. 2018;19(6):1641. https://doi.org/10.3390/ijms19061641.
Datta P, Vyas V, Dhara S, Chowdhury AR, Barui A. Anisotropy properties of tissues: a basis for fabrication of biomimetic anisotropic scaffolds for tissue engineering. J Bionic Eng. 2019;16(5):842–68. https://doi.org/10.1007/s42235-019-0101-9.
Hasan A, Waibhaw G, Saxena V, Pandey LM. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int J Biol Macromol. 2018;111:923–34. https://doi.org/10.1016/j.ijbiomac.2018.01.089.
Wanjale SD, Jog JP. Crystallization and phase transformation kinetics of poly (1-butene)/MWCNT nanocomposites. Polymer. 2006;47(18):6414–21. https://doi.org/10.1016/j.polymer.2006.07.011.
El Fray M, Boccaccini AR. Novel hybrid PET/DFA–TiO2 nanocomposites by in situ polycondensation. Mater Lett. 2005;59(18):2300–4. https://doi.org/10.1016/j.matlet.2005.03.008.
Liu A, Hong Z, Zhuang X, Chen X, Cui Y, Liu Y, Jing X. Surface modification of bioactive glass nanoparticles and the mechanical and biological properties of poly (L-lactide) composites. Acta Biomater. 2008;4(4):1005–15. https://doi.org/10.1016/j.actbio.2008.02.013.
Jawad H, Boccaccini AR, Ali NN, Harding SE. Assessment of cellular toxicity of TiO2 nanoparticles for cardiac tissue engineering applications. Nanotoxicology. 2011;5(3):372–80. https://doi.org/10.3109/17435390.2010.516844.
Lim D, Lee E, Kim H, Park S, Baek S, Yoon J. Multi stimuli-responsive hydrogel microfibers containing magnetite nanoparticles prepared using microcapillary devices. Soft Matter. 2015;11(8):1606–13. https://doi.org/10.1039/c4sm02564d.
Ghosh S, Webster TJ. Metallic Nanoscaffolds as osteogenic promoters: advances, challenges and scope. Metals. 2021;11(9):1356. https://doi.org/10.3390/met11091356.
Shevach M, Fleischer S, Shapira A, Dvir T. Gold nanoparticle-decellularized matrix hybrids for cardiac tissue engineering. Nano Lett. 2014;14(10):5792–6. https://doi.org/10.1021/nl502673m.
Shevach M, Maoz BM, Feiner R, Shapira A, Dvir T. Nanoengineering gold particle composite fibers for cardiac tissue engineering. J Mater Chem B. 2013;1(39):5210–7. https://doi.org/10.1039/c3tb20584c.
Navaei A, Saini H, Christenson W, Sullivan RT, Ros R, Nikkhah M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016;41:133–46. https://doi.org/10.1016/j.actbio.2016.05.027.
Kharaziha M, Memic A, Akbari M, Brafman DA, Nikkhah M. Nano-enabled approaches for stem cell-based cardiac tissue engineering. Adv Healthc Mater. 2016;5(13):1533–53. https://doi.org/10.1002/adhm.201600088.
Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold nanoparticles for biology and medicine. Angew Chem Int Ed Engl. 2010;49(19):3280–94. https://doi.org/10.1002/anie.200904359.
Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev. 2012;41(6):2256–82. https://doi.org/10.1039/c1cs15166e.
Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38(6):1759–82. https://doi.org/10.1039/b806051g.
Chen Y, Li N, Yang Y, Liu Y. A dual targeting cyclodextrin/gold nanoparticle conjugate as a scaffold for solubilization and delivery of paclitaxel. RSC Adv. 2015;5(12):8938–41. https://doi.org/10.1039/C4RA13135E.
Ko WK, Heo DN, Moon HJ, Lee SJ, Bae MS, Lee JB, Sun IC, Jeon HB, Park HK, Kwon IK. The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. J Colloid Interface Sci. 2015;438:68–76. https://doi.org/10.1016/j.jcis.2014.08.058.
Suh KS, Lee YS, Seo SH, Kim YS, Choi EM. Gold nanoparticles attenuates antimycin A-induced mitochondrial dysfunction in MC3T3-E1 osteoblastic cells. Biol Trace Elem Res. 2013;153(1):428–36. https://doi.org/10.1007/s12011-013-9679-7.
Motola M, Capek J, Zazpe R, Bacova J, Hromadko L, Bruckova L, Ng S, Handl J, Spotz Z, Knotek P, Baishya K. Thin TiO2 coatings by ALD enhance the cell growth on TiO2 nanotubular and flat substrates. ACS Appl Bio Mater. 2020;3(9):6447–56. https://doi.org/10.1021/acsabm.0c00871.
MacNeil S. Biomaterials for tissue engineering of skin. Mater Today. 2008;11(5):26–35. https://doi.org/10.1016/S1369-7021(08)70087-7.
Singla R, Abidi SM, Dar AI, Acharya A. Nanomaterials as potential and versatile platform for next generation tissue engineering applications. J Biomed Mater Res Part B Appl Biomater. 2019;107(7):2433–49. https://doi.org/10.1002/jbm.b.34327.
Sharif S, Ai J, Azami M, Verdi J, Atlasi MA, Shirian S, Samadikuchaksaraei A. Collagen-coated nano-electrospun PCL seeded with human endometrial stem cells for skin tissue engineering applications. J Biomed Mater Res B Appl Biomater. 2018;106:1578–86. https://doi.org/10.1002/jbm.b.33966.
Tocco I, Zavan B, Bassetto F, Vindigni V. Nanotechnology-based therapies for skin wound regeneration. J Nanomater. 2012;4:1–11. https://doi.org/10.1155/2012/714134.
Kaler A, Mittal AK, Katariya M, Harde H, Agrawal AK, Jain S, Banerjee UC. An investigation of in vivo wound healing activity of biologically synthesized silver nanoparticles. J Nanopart Res. 2014;16:2605–14. https://doi.org/10.1007/s11051-014-2605-x.
Rigo C, Ferroni L, Tocco I, Roman M, Munivrana I, Gardin C, Zavan B. Active silver nanoparticles for wound healing. Int J Mol Sci. 2013;14:4817–40. https://doi.org/10.3390/ijms14034817.
Liu J, Sonshine DA, Shervani S, Hurt RH. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano. 2010;4:6903–13. https://doi.org/10.1021/nn102272n.
Oyarzun-Ampuero F, Vidal A, Concha M, Morales J, Orellana S, Moreno VI. Nanoparticles for the treatment of wounds. Curr Pharm Des. 2015;21:4329–41. https://doi.org/10.2174/1381612821666150901104601.
Shi S, Jiang W, Zhao T, Aifantis KE, Wang H, Lin L, Fan Y, Feng Q, Cui FZ, Li X. The application of nanomaterials in controlled drug delivery for bone regeneration. J Biomed Mater Res A. 2015;103:3978–92. https://doi.org/10.1016/j.nano.2015.04.005.
Hasani-Sadrabadi MM, Hajrezaei SP, Emami SH, Bahlakeh G, Daneshmandi L, Dashtimoghadam E, Tayebi L. Enhanced osteogenic differentiation of stem cells via microfluidics synthesized nanoparticles. Nanomedicine: Nanotechnology Biology Medicine. 2015;11:1809 1819. https://doi.org/10.1016/j.nano.2015.04.005
Gentile P, Nandagiri VK, Pabari R, Daly J, Tonda-Turo C, Ciardelli G, Ramtoola Z. Influence of parathyroid hormone-loaded PLGA nanoparticles in porous scaffolds for bone regeneration. Int J Mol Sci. 2015;16:20492–510. https://doi.org/10.3390/ijms160920492.
Cao L, Werkmeister JA, Wang J, Glattauer V, McLean KM, Liu C. Bone regeneration using photocrosslinked hydrogel incorporating rhBMP-2 loaded 2-N, 6-O-sulfated chitosan nanoparticles. Biomaterials. 2014;35:2730–42. https://doi.org/10.1016/j.biomaterials.2013.12.028.
Venkatesan J, Pallela R, Kim SK. Applications of carbon nanomaterials in bone tissue engineering. J Biomed Nanotechnol. 2014;10:3105–23. https://doi.org/10.1166/jbn.2014.1969.
Chen W, Tsai PH, Hung Y, Chiou SH, Mou CY. Nonviral cell labelling and differentiation agent for induced pluripotent stem cells based on mesoporous silica nanoparticles. ACS Nano. 2013;7:8423–40. https://doi.org/10.1021/nn401418n.
Amin KA, Hashem KS, Alshehri FS, Awad ST, Hassan MS. Antioxidant and hepatoprotective efficiency of selenium nanoparticles against acetaminophen-induced hepatic damage. Biol Trace Elem Res. 2017;175:136–45. https://doi.org/10.1007/s12011-016-0748-6.
Pulavendran S, Rose C, Mandal AB. Hepatocyte growth factor incorporated chitosan nanoparticles augment the differentiation of stem cell into hepatocytes for the recovery of liver cirrhosis in mice. J Nanobiotechnol. 2011;9:15–25. https://doi.org/10.1186/1477-3155-9-15.
Hashemi SM, Soleimani M, Zargarian SS, Haddadi-Asl SV, Ahmadbeigi N, Soudi S, Mohammadi Y. In vitro differentiation of human cord blood-derived unrestricted somatic stem cells into hepatocyte-like cells on poly (ε-caprolactone) nanofiber scaffolds. Cells Tissues Organs. 2008;190:135–49. https://doi.org/10.1159/000187716.
Chen Z, Chang R, Guan W, Cai H, Tang F, Zhu W, Chen J. Transplantation of porcine hepatocytes cultured with polylactic acid-o-carboxymethylated chitosan nanoparticles promotes liver regeneration in acute liver failure rats. J Drug Deliv. 2011;2011:1–7. https://doi.org/10.1155/2011/797503.
Cui W, Zhou Y, Chang J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci Technol Adv Mater. 2016;11(014108): 014119. https://doi.org/10.1088/1468-6996/11/1/014108.
Agheb M, Dinari M, Rafienia M, Salehi H. Novel electrospun nanofibers of modified gelatin-tyrosine in cartilage tissue engineering. Mater Sci Eng C. 2017;71:240–51. https://doi.org/10.1016/j.msec.2016.10.003.
Naseri N, Poirier JM, Girandon L, Frohlich M, Oksman K, Mathew AP. 3-dimensional porous nanocomposite scaffolds based on cellulose nanofibers for cartilage tissue engineering: tailoring of porosity and mechanical performance. RSC Adv. 2016;6:5999–6007. https://doi.org/10.1039/C5RA27246G.
Zheng X, Wang W, Liu S, Wu J, Li F, Cao L, Fan C. Enhancement of chondrogenic differentiation of rabbit mesenchymal stem cells by oriented nanofiber yarn-collagen type I/hyaluronate hybrid. Mater Sci Eng C. 2016;58:1071–6. https://doi.org/10.1016/j.msec.2015.07.066.
Holmes B, Castro NJ, Li J, Keidar M, Zhang LG. Enhanced human bone marrow mesenchymal stem cell functions in novel 3D cartilage scaffolds with hydrogen treated multi-walled carbon nanotubes. Nanotechnology. 2013;24:365102–12. https://doi.org/10.1088/0957-4484/24/36/365102.
Kumar S, Chatterjee K. Comprehensive review on the use of graphene-based substrates for regenerative medicine and biomedical devices. ACS Appl Mater Interfaces. 2016;8:26431–57. https://doi.org/10.1021/acsami.6b09801.
Shalumon KT, Deepthi S, Anupama MS, Nair SV, Jayakumar R, Chennazhi KP. Fabrication of poly(l-lactic acid)/gelatin composite tubular scaffolds for vascular tissue engineering. Int J Biol Macromol. 2015;72:1048–55. https://doi.org/10.1016/j.ijbiomac.2014.09.058.
Fu W, Liu Z, Feng B, Hu R, He X, Wang H, Wang W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int J Nanomed. 2014;9:2335–44. https://doi.org/10.2147/IJN.S61375.
Miller DC, Haberstroh KM, Webster TJ. PLGA nanometer surface features manipulate fibronectin interactions for improved vascular cell adhesion. J Biomed Mater Res A. 2007;81:678–84. https://doi.org/10.1002/jbm.a.31093.
Ostdiek AM, Ivey JR, Grant DA, Gopaldas J, Grant SA. An in vivo study of a gold nanocomposite biomaterial for vascular repair. Biomaterials. 2015;65:175–83. https://doi.org/10.1016/j.biomaterials.2015.06.045.
Rahimnejad M, Nasrollahi Boroujeni N, Jahangiri S, Rabiee N, Rabiee M, Makvandi P, Akhavan O, Varma RS. Prevascularized micro-/nano-sized spheroid/bead aggregates for vascular tissue engineering. Nanomicro Lett. 2021;13(1):1–24. https://doi.org/10.1007/s40820-021-00697-1.
Nguyen AH, Marsh P, Schmiess-Heine L, Burke PJ, Lee A, Lee J, Cao H. Cardiac tissue engineering: state-of-the-art methods and outlook. J Biol Eng. 2019;13:57. https://doi.org/10.1186/s13036-019-0185-0.
Majid QA, Fricker ATR, Gregory DA, Davidenko N, Hernandez Cruz O, Jabbour RJ, Owen TJ, Basnett P, Lukasiewicz B, Stevens M, Best S, Cameron R, Sinha S, Harding SE, Roy I. Natural biomaterials for cardiac tissue engineering: a highly biocompatible solution. Front Cardiovasc Med. 2020;7: 554597. https://doi.org/10.3389/fcvm.2020.554597.
Montero P, Flandes-Iparraguirre M, Musquiz S, Pérez Araluce M, Plano D, Sanmartín C, Orive G, Gavira JJ, Prosper F, Mazo MM. Cells, materials, and fabrication processes for cardiac tissue engineering. Front Bioeng Biotechnol. 2020;8:955. https://doi.org/10.3389/fbioe.2020.00955.
Radhakrishnan S, Nagarajan S, Belaid H, Farha C, Iatsunskyi I, Coy E, Soussan L, Huon V, Bares J, Belkacemi K, Teyssier C. Fabrication of 3D printed antimicrobial polycaprolactone scaffolds for tissue engineering applications. Mater Sci Eng C. 2021;118: 111525. https://doi.org/10.1016/j.msec.2020.111525.
Dhand C, Balakrishnan Y, Ong ST, Dwivedi N, Venugopal JR, Harini S, Leung CM, Low KZW, Loh XJ, Beuerman RW, Ramakrishna S, Verma NK, Lakshminarayanan R. Antimicrobial quaternary ammonium organosilane cross-linked nanofibrous collagen scaffolds for tissue engineering. Int J Nanomedicine. 2018;13:4473–92. https://doi.org/10.2147/IJN.S159770.
De Silva RT, Dissanayake RK, Mantilaka MP, Wijesinghe WS, Kaleel SS, Premachandra TN, Weerasinghe L, Amaratunga GA, de Silva KN. Drug-loaded halloysite nanotube-reinforced electrospun alginate-based nanofibrous scaffolds with sustained antimicrobial protection. ACS Appl Mater Interfaces. 2018;10(40):33913–22. https://doi.org/10.1021/acsami.8b11013.
Mondal S, Hoang G, Manivasagan P, Moorthy MS, Nguyen TP, Phan TT, Kim HH, Kim MH, Nam SY, Oh J. Nano-hydroxyapatite bioactive glass composite scaffold with enhanced mechanical and biological performance for tissue engineering application. Ceram Int. 2018;44(13):15735–46. https://doi.org/10.1016/j.ceramint.2018.05.248.
Mondal S, Nguyen TP, Hoang G, Manivasagan P, Kim MH, Nam SY, Oh J. Hydroxyapatite nano bioceramics optimized 3D printed poly lactic acid scaffold for bone tissue engineering application. Ceram Int. 2020;46(3):3443–55. https://doi.org/10.1016/j.ceramint.2019.10.057.
Ahmed MK, Ramadan R, El-Dek SI, Uskoković V. Complex relationship between alumina and selenium-doped carbonated hydroxyapatite as the ceramic additives to electrospun polycaprolactone scaffolds for tissue engineering applications. J Alloys Compd. 2019;801:70–81. https://doi.org/10.1016/j.jallcom.2019.06.013.
Funda G, Taschieri S, Bruno GA, Grecchi E, Paolo S, Girolamo D, Del Fabbro M. Nanotechnology scaffolds for alveolar bone regeneration. Materials (Basel). 2020;13(1):201. https://doi.org/10.3390/ma13010201.
••Eivazzadeh-Keihan R, Maleki A, de la Guardia M, Bani MS, Chenab KK, Pashazadeh-Panahi P, Baradaran B, Mokhtarzadeh A, Hamblin MR. Carbon based nanomaterials for tissue engineering of bone: building new bone on small black scaffolds: a review. J Adv Res. 2019;18:185–201. https://doi.org/10.1016/j.jare.2019.03.011. This article reviewed various carbon-based materials that could be useful in the development of nanoscaffolds for tissue engineering.
•Ma H, Feng C, Chang J, Wu C. 3D-printed bioceramic scaffolds: from bone tissue engineering to tumor therapy. Acta biomater. 2018;79:37–59. https://doi.org/10.1016/j.actbio.2018.08.026. This article demonstrated different types of material and doping elements that provide more advantages for the bone tissue engineering.
Biswal T. Biopolymers for tissue engineering applications: a review. Materials Today: Proceedings. 2021;41:397–402. https://doi.org/10.1016/j.matpr.2020.09.628.
Aadil KR, Nathani A, Sharma CS, Lenka N, Gupta P. Investigation of poly (vinyl) alcohol-gellan gum based nanofiber as scaffolds for tissue engineering applications. J Drug Deliv Sci Technol. 2019;54:101276.
Bakhshayesh ARD, Mostafavi E, Alizadeh E, Asadi N, Akbarzadeh A, Davaran S. Fabrication of three-dimensional scaffolds based on nano-biomimetic collagen hybrid constructs for skin tissue engineering. ACS Omega. 2018;3(8):8605–11.
Mohandesnezhad S, Pilehvar-Soltanahmadi Y, Alizadeh E, Goodarzi A, Davaran S, Khatamian M, Zarghami N, Samiei M, Aghazadeh M, Akbarzadeh A. In vitro evaluation of Zeolite-nHA blended PCL/PLA nanofibers for dental tissue engineering. Mater Chem Phys. 2020;252:123152.
Maji S, Agarwal T, Das J, Maiti TK. Development of gelatin/carboxymethyl chitosan/nano-hydroxyapatite composite 3D macroporous scaffold for bone tissue engineering applications. Carbohydr Polym. 2018;189:115–25. https://doi.org/10.1016/j.carbpol.2018.01.104.
Saini RK, Bagri LP, Bajpai AK. Nanosilver hydroxyapatite based antibacterial 3D scaffolds of gelatin/alginate/poly (vinyl alcohol) for bone tissue engineering applications. Colloids Surf B Biointerfaces. 2019;1:211–8. https://doi.org/10.1016/j.colsurfb.2019.01.064.
Christy PN, Basha SK, Kumari VS, Bashir AK, Maaza M, Kaviyarasu K, Arasu MV, Al-Dhabi NA, Ignacimuthu S. Biopolymeric nanocomposite scaffolds for bone tissue engineering applications–a review. J Drug Deliv Sci Technol. 2020;55: 101452. https://doi.org/10.1016/j.jddst.2019.101452.
Iravani S, Varma RS. Plants and plant-based polymers as scaffolds for tissue engineering. Green Chem. 2019;21(18):4839–67. https://doi.org/10.1039/C9GC02391G.
Ebhodaghe SO. Natural polymeric scaffolds for tissue engineering applications. J Biomater Sci Polym Ed. 2021;32(16):2144–94. https://doi.org/10.1080/09205063.2021.1958185.
Ferreira FV, Otoni CG, Kevin J, Barud HS, Lona LM, Cranston ED, Rojas OJ. Porous nanocellulose gels and foams: breakthrough status in the development of scaffolds for tissue engineering. Mater Today. 2020;37:126–41. https://doi.org/10.1016/j.mattod.2020.03.003.
Khalil HP, Jummaat F, Yahya EB, Olaiya NG, Adnan AS, Abdat M, NAM N, Halim AS, Kumar U, Bairwan R, Suriani AB. A review on micro-to nanocellulose biopolymer scaffold forming for tissue engineering applications. Polymers. 2020;12(9):2043. https://doi.org/10.3390/polym12092043
Siddiqui N, Asawa S, Birru B, Baadhe R, Rao S. PCL-based composite scaffold matrices for tissue engineering applications. Mol Biotechnol. 2018;60(7):506–32. https://doi.org/10.1007/s12033-018-0084-5.
Bakhtiari SS, Karbasi S, Toloue EB. Modified poly (3-hydroxybutyrate)-based scaffolds in tissue engineering applications: a review. Int J Biol Macromol. 2021;1(166):986–98. https://doi.org/10.1016/j.ijbiomac.2020.10.255.
Ahmed S, Ali A, Sheikh J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int J Biol Macromol. 2018;116:849–62. https://doi.org/10.1016/j.ijbiomac.2018.04.176.
Pandit AH, Mazumdar N, Ahmad S. Periodate oxidized hyaluronic acid-based hydrogel scaffolds for tissue engineering applications. Int J Biol Macromol. 2019;137:853–69. https://doi.org/10.1016/j.ijbiomac.2019.07.014.
Bai RG, Muthoosamy K, Manickam S, Hilal-Alnaqbi A. Graphene-based 3D scaffolds in tissue engineering: fabrication, applications, and future scope in liver tissue engineering. Int J Nanomedicine. 2019;14:5753–83. https://doi.org/10.2147/IJN.S192779.
Shadjou N, Hasanzadeh M, Khalilzadeh B. Graphene based scaffolds on bone tissue engineering. Bioengineered. 2018;9(1):38–47. https://doi.org/10.1080/21655979.2017.1373539.
Bei HP, Yang Y, Zhang Q, Tian Y, Luo X, Yang M, Zhao X. Graphene-based nanocomposites for neural tissue engineering. Molecules. 2019;24(4):658. https://doi.org/10.3390/molecules24040658.
••Nalvuran H, Elçin AE, Elçin YM. Nanofibrous silk fibroin/reduced graphene oxide scaffolds for tissue engineering and cell culture applications. Int J Biol Macromol. 2018;114:77–84. https://doi.org/10.1016/j.ijbiomac.2018.03.072. This research article demonstrated the nanoscaffolds formed by reduced graphene oxide and silk fibroin and its properties against bone marrow–mesenchymal stem cells.
Acknowledgements
The authors are grateful to Chettinad Academy of Research and Education for supporting GA and DB with Junior Research Fellowship. We also offer sincere thanks to the Council of Scientific and Industrial Research (CSIR), India, for the grant with Scheme No. 01(2868)/17/EMR-II.
Funding
The research was supported by the Council of Scientific and Industrial Research (CSIR) India, Scheme No. 01(2868)/17/EMR-II. GA and DB were supported by Junior Research Fellowship from Chettinad Academy of Research and Education.
Author information
Authors and Affiliations
Contributions
All the authors have equally contributed for concept designing, literature searching, and manuscript writing. KG and AG finalized the draft.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All the authors have given the consent to publish.
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Naturopathy, Nanotechnology, Nutraceuticals, and Immunotherapy in Cancer Research
Rights and permissions
About this article
Cite this article
Deepika, B., Gopikrishna, A., Girigoswami, A. et al. Applications of Nanoscaffolds in Tissue Engineering. Curr Pharmacol Rep 8, 171–187 (2022). https://doi.org/10.1007/s40495-022-00284-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-022-00284-x