Abstract
Background
Engaging in both resistance and endurance exercise within the same training program, termed ‘concurrent exercise training,’ is common practice in many athletic disciplines that require a combination of strength and endurance and is recommended by a number of organizations to improve muscular and cardiovascular health and reduce the risk of chronic metabolic disease. Dietary protein ingestion supports skeletal muscle remodeling after exercise by stimulating the synthesis of muscle proteins and can optimize resistance exercise-training mediated increases in skeletal muscle size and strength; however, the effects of protein supplementation on acute and longer-term adaptive responses to concurrent resistance and endurance exercise are unclear.
Objectives
The purpose of this systematic review is to evaluate the effects of dietary protein supplementation on acute changes in muscle protein synthesis and longer-term changes in muscle mass, strength, and aerobic capacity in responses to concurrent resistance and endurance exercise in healthy adults.
Methods
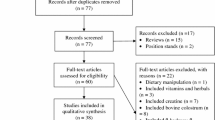
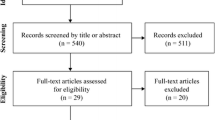
A systematic search was conducted in five databases: Scopus, Embase, Medline, PubMed, and Web of Science. Acute and longer-term controlled trials involving concurrent exercise and protein supplementation in healthy adults (ages 18–65 years) were included in this systematic review. Main outcomes of interest were changes in skeletal muscle protein synthesis rates, muscle mass, muscle strength, and whole-body aerobic capacity (i.e., maximal/peak aerobic capacity [VO2max/peak]). The quality of studies was assessed using the National Institute of Health Quality Assessment for Controlled Intervention Studies.
Results
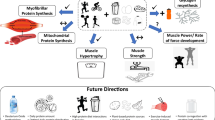
Four acute studies including 84 trained young males and ten longer-term studies including 167 trained and 391 untrained participants fulfilled the eligibility criteria. All included acute studies demonstrated that protein ingestion enhanced myofibrillar protein synthesis rates, but not mitochondrial protein synthesis rates during post-exercise recovery after an acute bout of concurrent exercise. Of the included longer-term training studies, five out of nine reported that protein supplementation enhanced concurrent training-mediated increases in muscle mass, while five out of nine studies reported that protein supplementation enhanced concurrent training-mediated increases in muscle strength and/or power. In terms of aerobic adaptations, all six included studies reported no effect of protein supplementation on concurrent training-mediated increases in VO2max/peak.
Conclusion
Protein ingestion after an acute bout of concurrent exercise further increases myofibrillar, but not mitochondrial, protein synthesis rates during post-exercise recovery. There is some evidence that protein supplementation during longer-term training further enhances concurrent training-mediated increases in skeletal muscle mass and strength/power, but not whole-body aerobic capacity (i.e., VO2max/peak).


Similar content being viewed by others
References
Coffey VG, Hawley JA. Concurrent exercise training: do opposites distract? J Physiol (Lond). 2017;595:2883–96.
Shamim B, Devlin BL, Timmins RG, Tofari P, Lee Dow C, Coffey VG, et al. Adaptations to concurrent training in combination with high protein availability: a comparative trial in healthy, recreationally active men. Sports Med. 2018;48:2869–83.
Glowacki SP, Martin SE, Maurer A, Baek W, Green JS, Crouse SF. Effects of resistance, endurance, and concurrent exercise on training outcomes in men. Med Sci Sports Exerc. 2004;36:2119–27.
Hawley JA. Specificity of training adaptation: time for a rethink? J Physiol. 2008;586:1–2.
Hawley JA. Adaptations of skeletal muscle to prolonged, intense endurance training. Clin Exp Pharmacol Physiol. 2002;29:218–22.
Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162–84.
Folland JP, Williams AG. The adaptations to strength training : morphological and neurological contributions to increased strength. Sports Med. 2007;37:145–68.
Damas F, Phillips S, Vechin F, Ugrinowitsch C. A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med (Auckland, NZ). 2015;2015:45.
McDonagh MJ, Davies CT. Adaptive response of mammalian skeletal muscle to exercise with high loads. Eur J Appl Physiol Occup Physiol. 1984;52:139–55.
Fyfe JJ, Bishop DJ, Stepto NK. Interference between concurrent resistance and endurance exercise: molecular bases and the role of individual training variables. Sports Med. 2014;44:743–62.
Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol. 1984;56:831–8.
Ruiz JR, Sui X, Lobelo F, Morrow JR, Jackson AW, Sjöström M, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ [Internet]. British Medical Journal Publishing Group. 2008. p. 337. https://www.bmj.com/content/337/bmj.a439. Accessed 25 Jun 2020.
Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl 3):1–72.
World Health Organization. Global Recommendations on Physical Activity for Health [Internet]. World Health Organization; 2010. https://www.who.int/dietphysicalactivity/global-PA-recs-2010.pdf.
Haskell WL, Lee I-M, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34.
Canadian Society for Exercise Physiology. Canadian Physical Activity Guidelines [Internet]. 2011. http://www.csep.ca/CMFiles/Guidelines/CSEP_PAGuidelinesQ&A_E.pdf.
Methenitis S. A brief review on concurrent training: from laboratory to the field. Sports (Basel) [Internet]; 2018. p. 6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6315763/. Accessed 25 Jun 2020.
Hickson RC. Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol. 1980;45:255–63.
Wilson JM, Marin PJ, Rhea MR, Wilson SMC, Loenneke JP, Anderson JC. Concurrent training: a meta-analysis examining interference of aerobic and resistance exercises. J Strength Cond Res. 2012;26:2293–307.
Häkkinen K, Alen M, Kraemer WJ, Gorostiaga E, Izquierdo M, Rusko H, et al. Neuromuscular adaptations during concurrent strength and endurance training versus strength training. Eur J Appl Physiol. 2003;89:42–52.
Hendrickson NR, Sharp MA, Alemany JA, Walker LA, Harman EA, Spiering BA, et al. Combined resistance and endurance training improves physical capacity and performance on tactical occupational tasks. Eur J Appl Physiol. 2010;109:1197–208.
Lundberg TR, Fernandez-Gonzalo R, Tesch PA. Exercise-induced AMPK activation does not interfere with muscle hypertrophy in response to resistance training in men. J Appl Physiol. 2014;116:611–20.
McCarthy JP, Pozniak MA, Agre JC. Neuromuscular adaptations to concurrent strength and endurance training. Med Sci Sports Exerc. 2002;34:511–9.
Sale DG, Jacobs I, MacDougall JD, Garner S. Comparison of two regimens of concurrent strength and endurance training. Med Sci Sports Exerc. 1990;22:348–56.
Sillanpää E, Häkkinen A, Nyman K, Mattila M, Cheng S, Karavirta L, et al. Body composition and fitness during strength and/or endurance training in older men. Med Sci Sports Exerc. 2008;40:950–8.
Sillanpää E, Laaksonen DE, Häkkinen A, Karavirta L, Jensen B, Kraemer WJ, et al. Body composition, fitness, and metabolic health during strength and endurance training and their combination in middle-aged and older women. Eur J Appl Physiol. 2009;106:285–96.
Da Silva R, Cadore E, Kothe G, Guedes M, Alberton C, Pinto S, et al. Concurrent training with different aerobic exercises. Int J Sports Med. 2012;33:627–34.
de Souza EO, Tricoli V, Roschel H, Brum PC, Bacurau AVN, Ferreira JCB, et al. Molecular adaptations to concurrent training. Int J Sports Med. 2013;34:207–13.
Tsitkanou S, Spengos K, Stasinaki A-N, Zaras N, Bogdanis G, Papadimas G, et al. Effects of high-intensity interval cycling performed after resistance training on muscle strength and hypertrophy. Scand J Med Sci Sports. 2017;27:1317–27.
Balabinis CP, Psarakis CH, Moukas M, Vassiliou MP, Behrakis PK. Early phase changes by concurrent endurance and strength training. J Strength Cond Res. 2003;17:393–401.
Lundberg TR, Fernandez-Gonzalo R, Gustafsson T, Tesch PA. Aerobic exercise does not compromise muscle hypertrophy response to short-term resistance training. J Appl Physiol. 1985;2013(114):81–9.
McCarthy JP, Agre JC, Graf BK, Pozniak MA, Vailas AC. Compatibility of adaptive responses with combining strength and endurance training. Med Sci Sports Exerc. 1995;27:429–36.
Murach KA, Bagley JR. Skeletal muscle hypertrophy with concurrent exercise training: contrary evidence for an interference effect. Sports Med. 2016;46:1029–39.
Jäger R, Kerksick CM, Campbell BI, Cribb PJ, Wells SD, Skwiat TM, et al. International society of sports nutrition position stand: protein and exercise. J Int Soc Sports Nutr. 2017;14:20.
Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol (Lond). 2009;587:897–904.
Burd NA, West DWD, Moore DR, Atherton PJ, Staples AW, Prior T, et al. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr. 2011;141:568–73.
Burd NA, West DWD, Staples AW, Atherton PJ, Baker JM, Moore DR, et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS ONE. 2010;5:e12033.
Churchward-Venne TA, Burd NA, Mitchell CJ, West DWD, Philp A, Marcotte GR, et al. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol (Lond). 2012;590:2751–65.
Burd NA, Gorissen SH, van Vliet S, Snijders T, van Loon LJ. Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: a randomized controlled trial. Am J Clin Nutr. 2015;102:828–36.
van Vliet S, Shy EL, Abou Sawan S, Beals JW, West DW, Skinner SK, et al. Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am J Clin Nutr. 2017;106:1401–12.
Hamarsland H, Nordengen AL, Nyvik Aas S, Holte K, Garthe I, Paulsen G, et al. Native whey protein with high levels of leucine results in similar post-exercise muscular anabolic responses as regular whey protein: a randomized controlled trial. J Int Soc Sports Nutr. 2017;14:43.
Churchward-Venne TA, Burd NA, Phillips SM. Nutritional regulation of muscle protein synthesis with resistance exercise: strategies to enhance anabolism. Nutr Metab. 2012;9:40.
Churchward-Venne TA, Pinckaers PJM, Smeets JSJ, Betz MW, Senden JM, Goessens JPB, et al. Dose-response effects of dietary protein on muscle protein synthesis during recovery from endurance exercise in young men: a double-blind randomized trial. Am J Clin Nutr. 2020;112:303–17.
Breen L, Philp A, Witard OC, Jackman SR, Selby A, Smith K, et al. The influence of carbohydrate–protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. J Physiol. 2011;589:4011–25.
Coffey VG, Moore DR, Burd NA, Rerecich T, Stellingwerff T, Garnham AP, et al. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Eur J Appl Physiol. 2011;111:1473–83.
Levenhagen DK, Carr C, Carlson MG, Maron DJ, Borel MJ, Flakoll PJ. Postexercise protein intake enhances whole-body and leg protein accretion in humans. Med Sci Sports Exerc. 2002;34:828–37.
Philp A, Hamilton DL, Baar K. Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J Appl Physiol. 1985;2011(110):561–8.
Baar K. Using molecular biology to maximize concurrent training. Sports Med. 2014;44:117–25.
Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mTOR signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care. 2008;11:222–6.
Phillips SM. The impact of protein quality on the promotion of resistance exercise-induced changes in muscle mass. Nutr Metab. 2016;13:64.
Phillips SM. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med. 2014;44:71–7.
Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol Am Physiol Soc. 2009;106:1692–701.
Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr Oxf Acad. 2012;96:1454–64.
Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine; 2018;52:376–84.
Ferguson-Stegall L, McCleave E, Ding Z, Doerner Iii PG, Liu Y, Wang B, et al. Aerobic exercise training adaptations are increased by postexercise carbohydrate-protein supplementation. J Nutr Metab. 2011;2011:623182.
Knuiman P, van Loon LJC, Wouters J, Hopman M, Mensink M. Protein supplementation elicits greater gains in maximal oxygen uptake capacity and stimulates lean mass accretion during prolonged endurance training: a double-blind randomized controlled trial. Am J Clin Nutr. 2019;110:508–18.
Bassi D, Mendes RG, Arakelian VM, Caruso FCR, Cabiddu R, Júnior JCB, et al. Potential effects on cardiorespiratory and metabolic status after a concurrent strength and endurance training program in diabetes patients—a randomized controlled trial. Sports Med Open. 2016;2:31.
Atashak S, Stannard SR, Azizbeigi K. Cardiovascular risk factors adaptation to concurrent training in overweight sedentary middle-aged men. J Sports Med Phys Fitness. 2016;56:624–30.
Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 2017;25:581–92.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. Public Library of Science. 2009;6:e1000097.
Hamilton DL, Philp A. Can AMPK mediated suppression of mTORC1 explain the concurrent training effect? Cell Mol Exerc Physiol. 2013;2:e4.
Study Quality Assessment Tools|NHLBI, NIH [Internet]. 2021. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 8 May 2021.
Beelen M, Tieland M, Gijsen AP, Vandereyt H, Kies AK, Kuipers H, et al. Coingestion of carbohydrate and protein hydrolysate stimulates muscle protein synthesis during exercise in young men, with no further increase during subsequent overnight recovery. J Nutr. 2008;138:2198–204.
Camera DM, West DWD, Phillips SM, Rerecich T, Stellingwerff T, Hawley JA, et al. Protein ingestion increases myofibrillar protein synthesis after concurrent exercise. Med Sci Sports Exerc. 2015;47:82–91.
Churchward-Venne TA, Pinckaers PJM, Smeets JSJ, Peeters WM, Zorenc AH, Schierbeek H, et al. Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with milk protein, whey, or micellar casein after concurrent resistance- and endurance-type exercise. J Nutr. 2019;149:198–209.
Parr EB, Camera DM, Areta JL, Burke LM, Phillips SM, Hawley JA, et al. Alcohol ingestion impairs maximal post-exercise rates of myofibrillar protein synthesis following a single bout of concurrent training. PLoS One. Public Library of Science; 2014;9:e88384.
Arciero PJ, Ives SJ, Norton C, Escudero D, Minicucci O, O’Brien G, et al. Protein-pacing and multi-component exercise training improves physical performance outcomes in exercise-trained women: the PRISE 3 study. Nutrients [Internet]. 2016; p. 8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4924173/. Accessed 16 Jun 2020.
Forbes SC, Bell GJ. Whey protein isolate or concentrate combined with concurrent training does not augment performance, cardiorespiratory fitness, or strength adaptations. J Sports Med Phys Fitness. 2020;60:832–40.
Ives SJ, Norton C, Miller V, Minicucci O, Robinson J, O’Brien G, et al. Multi-modal exercise training and protein-pacing enhances physical performance adaptations independent of growth hormone and BDNF but may be dependent on IGF-1 in exercise-trained men. Growth Horm IGF Res. 2017;32:60–70.
Jendricke P, Kohl J, Centner C, Gollhofer A, König D. Influence of specific collagen peptides and concurrent training on cardiometabolic parameters and performance indices in women: a randomized controlled trial. Front Nutr [Internet]. Frontiers; 2020. https://www.frontiersin.org/articles/10.3389/fnut.2020.580918/full. Accessed 20 Dec 2020.
Cronin O, Barton W, Skuse P, Penney NC, Garcia-Perez I, Murphy EF, et al. A prospective metagenomic and metabolomic analysis of the impact of exercise and/or whey protein supplementation on the gut microbiome of sedentary adults. mSystems. 2018;3:e00044-18.
Lockwood CM, Moon JR, Tobkin SE, Walter AA, Smith AE, Dalbo VJ, et al. Minimal nutrition intervention with high-protein/low-carbohydrate and low-fat, nutrient-dense food supplement improves body composition and exercise benefits in overweight adults: a randomized controlled trial. Nutr Metab (Lond). 2008;5:11.
Ormsbee MJ, Willingham BD, Marchant T, Binkley TL, Specker BL, Vukovich MD. Protein supplementation during a 6-month concurrent training program: effect on body composition and muscular strength in sedentary individuals. Int J Sport Nutr Exerc Metabol Hum Kinet. 2018;28:619–28.
Weinheimer EM, Conley TB, Kobza VM, Sands LP, Lim E, Janle EM, et al. Whey protein supplementation does not affect exercise training-induced changes in body composition and indices of metabolic syndrome in middle-aged overweight and obese adults. J Nutr. 2012;142:1532–9.
Walker TB, Smith J, Herrera M, Lebegue B, Pinchak A, Fischer J. The influence of 8 weeks of whey-protein and leucine supplementation on physical and cognitive performance. Int J Sport Nutr Exerc Metabol Hum Kinet Inc 2010;20:409–17.
Gryson C, Ratel S, Rance M, Penando S, Bonhomme C, Le Ruyet P, et al. Four-month course of soluble milk proteins interacts with exercise to improve muscle strength and delay fatigue in elderly participants. J Am Med Dir Assoc. 2014;15:958.e1-958.e9.
Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–83.
Moore DR. Maximizing post-exercise anabolism: the case for relative protein intakes. Front Nutr [Internet]. Frontiers; 2019. p. 6. https://www.frontiersin.org/articles/10.3389/fnut.2019.00147/full. Accessed 17 Jul 2020.
Trommelen J, Betz MW, van Loon LJC. The muscle protein synthetic response to meal ingestion following resistance-type exercise. Sports Med. 2019;49:185–97.
Donges CE, Burd NA, Duffield R, Smith GC, West DWD, Short MJ, et al. Concurrent resistance and aerobic exercise stimulates both myofibrillar and mitochondrial protein synthesis in sedentary middle-aged men. J Appl Physiol. 2012;112:1992–2001.
Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89:161–8.
Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr. 2014;99:86–95.
Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70:57–62.
Howarth KR, Phillips SM, MacDonald MJ, Richards D, Moreau NA, Gibala MJ. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. J Appl Physiol. 1985;2010(109):431–8.
Moore D, Camera D, Areta J, Hawley J. Beyond muscle hypertrophy: why dietary protein is important for endurance athletes. Appl Physiol Nutr Metabol. 2014;39:1–11.
Tarnopolsky M. Protein requirements for endurance athletes. Nutrition. 2004;20:662–8.
Tarnopolsky M. Protein requirements for endurance athletes. Eur J Sport Sci Routl. 2004;4:1–15.
Felig P, Wahren J. Amino acid metabolism in exercising man. J Clin Invest. 1971;50:2703–14.
Haralambie G, Berg A. Serum urea and amino nitrogen changes with exercise duration. Eur J Appl Physiol. 1976;36:39–48.
Lamont LS, McCullough AJ, Kalhan SC. Relationship between leucine oxidation and oxygen consumption during steady-state exercise. Med Sci Sports Exerc. 2001;33:237–41.
Lemon PW, Mullin JP. Effect of initial muscle glycogen levels on protein catabolism during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1980;48:624–9.
Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA. 1997;94:14930–5.
Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, et al. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;280:E340-348.
Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, et al. The rate of protein digestion affects protein gain differently during aging in humans. J Physiol. 2003;549:635–44.
Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. 1985;2009(107):987–92.
Pennings B, Boirie Y, Senden JMG, Gijsen AP, Kuipers H, van Loon LJC. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011;93:997–1005.
Yang Y, Churchward-Venne TA, Burd NA, Breen L, Tarnopolsky MA, Phillips SM. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond). 2012;9:57.
Tipton KD, Elliott TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc. 2004;36:2073–81.
Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, et al. Whey and casein labeled with L-[1-13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am J Physiol Endocrinol Metab. 2011;300:E231-242.
Dideriksen KJ, Reitelseder S, Petersen SG, Hjort M, Helmark IC, Kjaer M, et al. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scand J Med Sci Sports. 2011;21:e372-383.
Churchward-Venne TA, Pinckaers PJM, Smeets JSJ, Peeters WM, Zorenc AH, Schierbeek H, et al. Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with whey, soy, or leucine-enriched soy protein after concurrent resistance- and endurance-type exercise. J Nutr. 2019;149:210–20.
Abou Sawan S, van Vliet S, Parel JT, Beals JW, Mazzulla M, West DWD, et al. Translocation and protein complex co‐localization of mTOR is associated with postprandial myofibrillar protein synthesis at rest and after endurance exercise. Physiol Rep [Internet]; 2018. p. 6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5840389/. Accessed 28 Jul 2020.
Bohé J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–9.
Bohé J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol. 2003;552:315–24.
Beals JW, Mackenzie RWA, van Vliet S, Skinner SK, Pagni BA, Niemiro GM, et al. Protein-rich food ingestion stimulates mitochondrial protein synthesis in sedentary young adults of different BMIs. J Clin Endocrinol Metab. 2017;102:3415–24.
Di Donato DM, West DWD, Churchward-Venne TA, Breen L, Baker SK, Phillips SM. Influence of aerobic exercise intensity on myofibrillar and mitochondrial protein synthesis in young men during early and late postexercise recovery. Am J Physiol Endocrinol Metab. 2014;306:E1025-1032.
Hansen M, Oxfeldt M, Larsen AE, Thomsen LS, Rokkedal-Lausch T, Christensen B, et al. Supplement with whey protein hydrolysate in contrast to carbohydrate supports mitochondrial adaptations in trained runners. J Int Soc Sports Nutr. 2020;17:46.
Holwerda AM, Bouwman FG, Nabben M, Wang P, van Kranenburg J, Gijsen AP, et al. Endurance-type exercise increases bulk and individual mitochondrial protein synthesis rates in rats. Int J Sport Nutr Exerc Metab. 2020;2020:1–12.
Hesketh SJ, Stansfield BN, Stead CA, Burniston JG. The application of proteomics in muscle exercise physiology. Expert Rev Proteomics. 2020;17:813–25.
Karlsson HKR, Nilsson P-A, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E. Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab. 2004;287:E1-7.
Koopman R, Pennings B, Zorenc AHG, van Loon LJC. Protein ingestion further augments S6K1 phosphorylation in skeletal muscle following resistance type exercise in males. J Nutr Oxf Acad. 2007;137:1880–6.
Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, et al. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol-Endocrinol Metabol. American Physiological Society; 2008;294:E392–400.
Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, et al. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–62.
Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–4.
Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, et al. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr. 2010;92:1080–8.
Vissing K, McGee SL, Farup J, Kjølhede T, Vendelbo MH, Jessen N. Differentiated mTOR but not AMPK signaling after strength vs endurance exercise in training-accustomed individuals. Scand J Med Sci Sports. 2013;23:355–66.
Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, et al. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586:3701–17.
Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, et al. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006;20:190–2.
Murlasits Z, Kneffel Z, Thalib L. The physiological effects of concurrent strength and endurance training sequence: a systematic review and meta-analysis. J Sports Sci Routl. 2018;36:1212–9.
Timmons JA. Variability in training-induced skeletal muscle adaptation. J Appl Physiol. 1985;2011(110):846–53.
Atherton PJ, Smith K. Muscle protein synthesis in response to nutrition and exercise. J Physiol. 2012;590:1049–57.
Knuiman P, Hopman MTE, Wouters JA, Mensink M. Select skeletal muscle mRNAs related to exercise adaptation are minimally affected by different pre-exercise meals that differ in macronutrient profile. Front Physiol [Internet]; 2018. p. 9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5791349/. Accessed 21 Dec 2020.
Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–84.
Baar K. Nutrition and the adaptation to endurance training. Sports Med. 2014;44:5–12.
Pasiakos SM, Carbone JW. Assessment of skeletal muscle proteolysis and the regulatory response to nutrition and exercise. IUBMB Life. 2014;66:478–84.
Tipton KD, Hamilton DL, Gallagher IJ. Assessing the role of muscle protein breakdown in response to nutrition and exercise in humans. Sports Med. 2018;48:53–64.
Camera DM, Ong JN, Coffey VG, Hawley JA. Selective modulation of MicroRNA expression with protein ingestion following concurrent resistance and endurance exercise in human skeletal muscle. Front Physiol. 2016;7:87.
Sanchez AMJ, Candau RB, Bernardi H. FoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasis. Cell Mol Life Sci. 2014;71:1657–71.
Lira VA, Benton CR, Yan Z, Bonen A. PGC-1α regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;299:E145–61.
Olesen J, Kiilerich K, Pilegaard H. PGC-1alpha-mediated adaptations in skeletal muscle. Pflugers Arch. 2010;460:153–62.
Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–8.
Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, et al. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-γ Coactivator-1 and peroxisome proliferator-activated receptor-α in skeletal muscle. Diabetes Am Diabetes Assoc. 2003;52:2874–81.
Skovgaard C, Brandt N, Pilegaard H, Bangsbo J. Combined speed endurance and endurance exercise amplify the exercise-induced PGC-1α and PDK4 mRNA response in trained human muscle. Physiol Rep. 2016;2016:4.
Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ. Acute endurance exercise increases the nuclear abundance of PGC-1alpha in trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2010;298:R912-917.
Sun X, Zemel MB. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr Metab (Lond). 2009;6:26.
Smiles WJ, Parr EB, Coffey VG, Lacham-Kaplan O, Hawley JA, Camera DM. Protein coingestion with alcohol following strenuous exercise attenuates alcohol-induced intramyocellular apoptosis and inhibition of autophagy. Am J Physiol Endocrinol Metab. 2016;311:E836–49.
Hill KM, Stathis CG, Grinfeld E, Hayes A, McAinch AJ. Co-ingestion of carbohydrate and whey protein isolates enhance PGC-1α mRNA expression: a randomised, single blind, cross over study. J Int Soc Sports Nutr. 2013;10:8.
Russell AP, Lamon S, Boon H, Wada S, Güller I, Brown EL, et al. Regulation of miRNAs in human skeletal muscle following acute endurance exercise and short-term endurance training. J Physiol. 2013;591:4637–53.
Nielsen S, Scheele C, Yfanti C, Akerström T, Nielsen AR, Pedersen BK, et al. Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J Physiol. 2010;588:4029–37.
Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, et al. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol. 1985;2011(110):309–17.
Kirby TJ, McCarthy JJ. MicroRNAs in skeletal muscle biology and exercise adaptation. Free Radic Biol Med. 2013;64:95–105.
He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31.
Margolis LM, McClung HL, Murphy NE, Carrigan CT, Pasiakos SM. Skeletal muscle myomiR are differentially expressed by endurance exercise mode and combined essential amino acid and carbohydrate supplementation. Front Physiol. 2017;8:182.
D’Souza RF, Zeng N, Markworth JF, Figueiredo VC, Hedges CP, Petersen AC, et al. Whey protein supplementation post resistance exercise in elderly men induces changes in muscle miRNA’s compared to resistance exercise alone. Front Nutr. 2019;6:91.
White AT, Schenk S. NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab. 2012;303:E308-321.
Gäbler M, Prieske O, Hortobágyi T, Granacher U. The effects of concurrent strength and endurance training on physical fitness and athletic performance in youth: a systematic review and meta-analysis. Front Physiol [Internet]. Frontiers; 2018. p. 9. https://www.frontiersin.org/articles/10.3389/fphys.2018.01057/full. Accessed 26 Jun 2020.
Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BBL, Verdijk LB, et al. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One. 2015;10:e0140903.
Schoenfeld BJ, Ogborn D, Krieger JW. Effects of resistance training frequency on measures of muscle hypertrophy: a systematic review and meta-analysis. Sports Med. 2016;46:1689–97.
Lin Y-N, Tseng T-T, Knuiman P, Chan WP, Wu S-H, Tsai C-L, et al. Protein supplementation increases adaptations to endurance training: a systematic review and meta-analysis. Clin Nutr. 2021;40:3123–32.
Beals JW, Burd NA, Moore DR, van Vliet S. Obesity alters the muscle protein synthetic response to nutrition and exercise. Front Nutr [Internet]. 2019. p. 6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6584965/. Accessed 28 Sep 2020.
Damas F, Phillips S, Vechin FC, Ugrinowitsch C. A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med. 2015;45:801–7.
Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Szewczyk NJ, Greenhaff PL, et al. Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J. 2015;29:4485–96.
Pasiakos SM, McLellan TM, Lieberman HR. The effects of protein supplements on muscle mass, strength, and aerobic and anaerobic power in healthy adults: a systematic review. Sports Med. 2015;45:111–31.
Petré H, Hemmingsson E, Rosdahl H, Psilander N. Development of maximal dynamic strength during concurrent resistance and endurance training in untrained, moderately trained, and trained individuals: a systematic review and meta-analysis. Sports Med. 2021;51:991–1010.
Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol. 1997;273:E790-800.
Clark LA, Russ DW, Tavoian D, Arnold WD, Law TD, France CR, et al. Heterogeneity of the strength response to progressive resistance exercise training in older adults: Contributions of muscle contractility. Exp Gerontol. 2021;152:111437.
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64.
Timmons JF, Hone M, Cogan KE, Duffy O, Egan B. Increased leg strength after concurrent aerobic and resistance exercise training in older adults is augmented by a whole food-based high protein diet intervention. Front Sports Active Living. 2021;3:52.
Smith GI, Atherton P, Villareal DT, Frimel TN, Rankin D, Rennie MJ, et al. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS One. Public Library of Science; 2008;3:e1875.
Forbes SC, Bell GJ. Whey protein isolate supplementation while endurance training does not alter cycling performance or immune responses at rest or after exercise. Front Nutr [Internet]. 2019. p. 6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6406070/. Accessed 25 Aug 2020.
Roberson PA, Romero MA, Mumford PW, Osburn SC, Haun CT, Vann CG, et al. Protein supplementation throughout 10 weeks of progressive run training is not beneficial for time trial improvement. Front Nutr. 2018;5:97.
Jonvik KL, Paulussen KJM, Danen SL, Ceelen IJM, Horstman AM, Wardenaar FC, et al. Protein supplementation does not augment adaptations to endurance exercise training. Med Sci Sports Exerc. 2019;51:2041–9.
Robinson MM, Turner SM, Hellerstein MK, Hamilton KL, Miller BF. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J. 2011;25:3240–9.
Knuiman P, Hopman MTE, Verbruggen C, Mensink M. Protein and the adaptive response with endurance training: wishful thinking or a competitive edge? Front Physiol [Internet]. 2018. p. 9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5974122/. Accessed 28 Jul 2020.
Hansen M, Bangsbo J, Jensen J, Bibby BM, Madsen K. Effect of whey protein hydrolysate on performance and recovery of top-class orienteering runners. Int J Sport Nutr Exerc Metabol Human Kinetics, Inc.; 2015;25:97–109.
Williamson E, Kato H, Volterman KA, Suzuki K, Moore DR. The effect of dietary protein on protein metabolism and performance in endurance-trained males. Med Sci Sports Exerc. 2019;51:352–60.
Morton RW, McGlory C, Phillips SM. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front Physiol [Internet]. Frontiers; 2015. p. 6. https://www.frontiersin.org/articles/10.3389/fphys.2015.00245/full. Accessed 1 Jul 2021.
Kephart WC, Wachs TD, Thompson RM, Brooks Mobley C, Fox CD, McDonald JR, et al. Ten weeks of branched-chain amino acid supplementation improves select performance and immunological variables in trained cyclists. Amino Acids. 2016;48:779–89.
Acknowledgements
The authors thank Dr Donny M. Camera and Dr Matthew D. Vukovich for providing raw data from their studies for this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no specific funding for this work.
Conflicts of interest/Competing interests
Felicia A. Hartono, Patrick W. Martin-Arrowsmith, Wouter M. Peeters and Tyler A. Churchward-Venne declare that they have no conflicts of interest relevant to the content of this review.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Authors' contributions
TAC-V conceived the idea for the manuscript; FAH, PWM-A, and WMP performed the literature search; FAH and WMP performed data extraction; FAH drafted the manuscript; all authors critically revised and edited the manuscript; all authors read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hartono, F.A., Martin-Arrowsmith, P.W., Peeters, W.M. et al. The Effects of Dietary Protein Supplementation on Acute Changes in Muscle Protein Synthesis and Longer-Term Changes in Muscle Mass, Strength, and Aerobic Capacity in Response to Concurrent Resistance and Endurance Exercise in Healthy Adults: A Systematic Review. Sports Med 52, 1295–1328 (2022). https://doi.org/10.1007/s40279-021-01620-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-021-01620-9