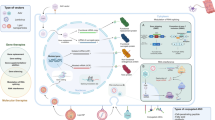
Abstract
Eladocagene exuparvovec (Upstaza™) is a gene therapy developed by PTC Therapeutics for the treatment of human aromatic L-amino acid decarboxylase (AADC) deficiency. Eladocagene exuparvovec comprises an adeno-associated virus vector that delivers the dopa decarboxylase (DDC) gene, the gene for human AADC. Eladocagene exuparvovec was approved in July 2022 in the EU for the treatment of patients aged 18 months and older with a clinical, molecular, and genetically confirmed diagnosis of AADC deficiency with a severe phenotype (i.e. patients who cannot sit, stand or walk). This article summarizes the milestones in the development of eladocagene exuparvovec leading to this first approval for the treatment of patients aged 18 months and older with AADC deficiency.
Similar content being viewed by others
References
Wassenberg T, Molero-Luis M, Jeltsch K, et al. Consensus guideline for the diagnosis and treatment of aromatic L-amino acid decarboxylase (AADC) deficiency. Orphanet J Rare Dis. 2017. https://doi.org/10.1186/s13023-016-0522-z.
Himmelreich N, Montioli R, Bertoldi M, et al. Aromatic amino acid decarboxylase deficiency: molecular and metabolic basis and therapeutic outlook. Mol Genet Metab. 2019;127(1):12–22.
Hwu PW, Kiening K, Anselm I, et al. Gene therapy in the putamen for curing AADC deficiency and Parkinson’s disease. EMBO Mol Med. 2021;13(9): e14712.
National Organisation for Rare Disorders Inc. Aromatic L-amino acid decarboxylase deficiency. 2021. https://rarediseases.org/. Accessed 8 Aug 2022.
European Medicines Agency. Eladocagene exuparvovec: EU summary of product characteristics 2022. https://ec.europa.eu/health/documents/community-register/html/h1653.htm. Accessed 25 July 2022
Pearson TS, Gilbert L, Opladen T, et al. AADC deficiency from infancy to adulthood: symptoms and developmental outcome in an international cohort of 63 patients. J Inherit Metab Dis. 2020;43(5):1121–30.
Hwu WL, Muramatsu S, Tseng SH, et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency. Sci Transl Med. 2012;4(134):134ra61.
Chien YH, Lee NC, Tseng SH, et al. Efficacy and safety of AAV2 gene therapy in children with aromatic L-amino acid decarboxylase deficiency: an open-label, phase 1/2 trial. Lancet Child Adolesc Health. 2017;1(4):265–73.
ClearPoint Neuro. Smartflow® Cannula. 2022. https://www.clearpointneuro.com/biologics-drug-delivery/smartflow-cannula/. Accessed 5 Aug 2022.
PTC Therapeutics Inc. PTC Therapeutics successfully completes acquisition of Agilis Biotherapeutics [media release]. 23 Aug 2018. http://www.ptcbio.com.
PTC Therapeutics Inc., Aldevron LLC. PTC Therapeutics establishes strategic collaboration with Aldevron to support GMP plasmid manufacturing [media release]. 7 Oct 2019. http://www.ptcbio.com.
Agilis Biotherapeutics. Agilis Biotherapeutics, first gene therapy company selected for NIH Therapeutics for Rare and Neglected Diseases Program [media release]. 27 June 2016. http://www.agilisbio.com.
Agilis Biotherapeutics. Agilis Biotherapeutics and National Taiwan University enter into worldwide, exclusive license agreement for the gene therapy treatment of AADC deficiency [media release]. 28 Jan 2016. http://www.agilisbio.com.
PTC Therapeutics Inc. Form 10-K. 2022. https://ir.ptcbio.com/static-files/947329bc-49e1-4826-98f0-17387af6e00e. Accessed 3 Aug 2022.
Tai CH, Lee NC, Chien YH, et al. Long-term efficacy and safety of eladocagene exuparvovec in patients with AADC deficiency. Mol Ther. 2022;30(2):509–18.
Hwu PWL, Chien YH, Lee NC, et al. Improved motor function in children with AADC deficiency treated with eladocagene exuparvovec (PTC-AADC): compassionate use study [abstract no. 611]. Mol Ther. 2020;28(4):270–1.
PTC Therapeutics Inc. Upstaza™ granted marketing authorization by European Commission as first disease-modifying treatment for AADC deficiency [media release]. 20 July 2022. https://ir.ptcbio.com/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Susan J. Keam is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keam, S.J. Eladocagene Exuparvovec: First Approval. Drugs 82, 1427–1432 (2022). https://doi.org/10.1007/s40265-022-01775-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01775-3