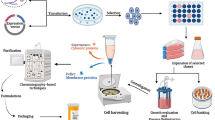
Abstract
Autoimmune diseases—where the immune system mistakenly targets self-tissue—remain hindered by non-specific therapies. For example, even molecularly specific monoclonal antibodies fail to distinguish between healthy cells and self-reactive cells. An experimental therapeutic approach involves delivery of self-molecules targeted by autoimmunity, along with immune modulatory signals to produce regulatory T cells (TREG) that selectively stop attack of host tissue. Much has been done to increase the efficiency of signal delivery using biomaterials, including encapsulation in polymer microparticles (MPs) to allow for co-delivery and cargo protection. However, less research has compared particles encapsulating drugs that target different TREG inducing pathways. In this paper, we use poly (lactic-co-glycolide) (PLGA) to co-encapsulate type 1 diabetes (T1D)-relevant antigen and 3 distinct TREG-inducing molecules — rapamycin (Rapa), all-trans retinoic acid (atRA), and butyrate (Buty) — that target the mechanistic target of Rapa (mTOR), the retinoid pathway, and histone deacetylase (HDAC) inhibition, respectively. We show all formulations are effectively taken up by antigen presenting cells (APCs) and that antigen-containing formulations are able to induce proliferation in antigen-specific T cells. Further, atRA and Rapa MP formulations co-loaded with antigen decrease APC activation levels, induce TREG differentiation, and reduce inflammatory cytokines in pancreatic-reactive T cells.
Graphical abstract







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article. All data files, Flowjo workspaces, and JMP files are available upon request.
Code availability
Not Applicable.
References
DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet (London, England). 2018;391:2449–62.
Cauwels A, Tavernier J. Tolerizing strategies for the treatment of autoimmune diseases: from ex vivo to in vivo strategies. Front Immunol. 2020;11.
Steinman L, Ho PP, Robinson WH, Utz PJ, Villoslada P. Antigen-specific tolerance to self-antigens in protein replacement therapy, gene therapy and autoimmunity. Curr Opin Immunol. 2019;61:46–53.
Gosselin EA, Eppler HB, Bromberg JS, Jewell CM. Designing natural and synthetic immune tissues. Nat Mater. 2018;17:484–98.
Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat Rev Immunol. 2008;8:607–18.
Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32.
Ferreira LMR, Muller YD, Bluestone JA, Tang Q. Next-generation regulatory T cell therapy. Nat Rev Drug Discovery. 2019;18:749–69.
Gammon JM, Jewell CM. Engineering immune tolerance with biomaterials. 2019. https://doi.org/10.1002/adhm.201801419.
Moorman CD, Sohn SJ, Phee H. Emerging therapeutics for immune tolerance: tolerogenic vaccines, T cell therapy, and IL-2 therapy. Front Immunol. 2021;12.
Marek-Trzonkowska N, et al. Administration of CD4+CD25highCD127- regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–20.
Duffy SS, Keating BA, Moalem-Taylor G. Adoptive transfer of regulatory T cells as a promising immunotherapy for the treatment of multiple sclerosis. Front Neurosci. 2019;13:1107.
Grimm AJ, Kontos S, Diaceri G, Quaglia-Thermes X, Hubbell JA. Memory of tolerance and induction of regulatory T cells by erythrocyte-targeted antigens. Sci Rep. 2015;5:15907.
Roep BO, et al. Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8 T cells in type 1 diabetes. Sci Translational Med. 2013;5:191ra82 LP-191ra82.
Van Y-H, et al. All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-γ{\textendash}producing T-cells without affecting Th17 cells. Diabetes. 2009;58:146–55.
Liu Z-M, Wang K-P, Ma J, Guo Zheng S. The role of all-trans retinoic acid in the biology of Foxp3+ regulatory T cells. Cellular & mol immunol. 2015;12:553–557.
Sun X, et al. All-trans retinoic acid induces CD4+CD25+FOXP3+ regulatory T cells by increasing FOXP3 demethylation in systemic sclerosis CD4+ T cells. J Immunol Res. 2018;2018:8658156.
Lu L, et al. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci. 2014;111:E3432–40.
Jacob N, et al. Butyrate induced Tregs are capable of migration from the GALT to the pancreas to restore immunological tolerance during type-1 diabetes. Sci Rep. 2020;10:19120.
Furusawa Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50.
Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–802.
Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015;36:3–12.
Tostanoski LH, et al. Reprogramming the local lymph node microenvironment promotes tolerance that is systemic and antigen specific. Cell Rep. 2016;16:2940–52.
Maldonado RA, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci. 2015;112:E156–65.
Froimchuk E, Carey ST, Edwards C, Jewell CM. Self-assembly as a molecular strategy to improve immunotherapy. Acc Chem Res. 2020. https://doi.org/10.1021/acs.accounts.0c00438.
Ben-Akiva E, Est Witte S, Meyer RA, Rhodes KR, Green JJ. Polymeric micro- and nanoparticles for immune modulation. Biomater sci. 2018;7:14–30.
Stabler CL, Li Y, Stewart JM, Keselowsky BG. Engineering immunomodulatory biomaterials for type 1 diabetes. Nat Rev Mater. 2019;4:429–50.
Bookstaver ML, Tsai SJ, Bromberg JS, Jewell CM. Improving vaccine and immunotherapy design using biomaterials. Trends Immunol. 2018;39:135–50.
Northrup L, Christopher MA, Sullivan BP, Berkland C. Combining antigen and immunomodulators: emerging trends in antigen-specific immunotherapy for autoimmunity. Adv Drug Deliv Rev. 2016;98:86–98.
Gosselin EA, Noshin M, Black SK, Jewell CM. Impact of excipients on stability of polymer microparticles for autoimmune therapy. Frontiers in Bioengineering and Biotechnology. 2021;8:1575.
Oakes RS, et al. Exploiting rational assembly to map distinct roles of regulatory cues during autoimmune therapy. ACS Nano. 2021;15:4305–20.
Tsai SJ, Amerman A, Jewell CM. Altering antigen charge to control self-assembly and processing of immune signals during cancer vaccination. https://doi.org/10.3389/fimmu.2020.613830.
Jewell CM, Bustamante López SC, Irvine DJ. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. https://doi.org/10.1073/pnas.1105200108/-/DCSupplemental.
Gosselin EA, Tostanoski LH, Jewell CM. Controlled release of second generation mTOR inhibitors to restrain inflammation in primary immune cells. AAPS Journal. 2017;19:1175–85.
Gammon JM, et al. Low-dose controlled release of mTOR inhibitors maintains T cell plasticity and promotes central memory T cells. J Control Release. 2017;263:151–61.
AU - Andork JI, AU - Tostanoski LH, AU - Solano E, AU - Mukhamedova M, AU - Jewell CM. Intra-lymph node injection of biodegradable polymer particles. JoVE. 2014;e50984. https://doi.org/10.3791/50984.
Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–100.
Gonzalez A, et al. Genetic Control of Diabetes Progression. Immunity. 1997;7:873–83.
Stadinski BD, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–31.
Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25:1815–21.
He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–66.
Mbongue JC, Nieves HA, Torrez TW, Langridge WHR. The role of dendritic cell maturation in the induction of insulin-dependent diabetes mellitus. Front Immunol. 2017;8:327.
Björck P, Flores-Romo L, Liu Y-J. Human interdigitating dendritic cells directly stimulate CD40-activated naive B cells. Eur J Immunol. 1997;27:1266–74.
Ye L, et al. mTOR promotes antiviral humoral immunity by differentially regulating CD4 helper T cell and B cell responses. J Virol. 2017;91.
Salmond RJ, Emery J, Okkenhaug K, Zamoyska R. MAPK, Phosphatidylinositol 3-kinase, and mammalian target of rapamycin pathways converge at the level of ribosomal protein S6 phosphorylation to control metabolic signaling in CD8 T cells. The J Immunol. 2009;183.
Iwata M, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2014;21.
Oakes RS, Froimchuk E, Jewell CM. Engineering biomaterials to direct innate immunity. Adv ther 2019;2.
Hess KL, Medintz IL, Jewell CM. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today. 2019;27:73–98.
Juang J-H, et al. Prevention and reversal of diabetes by all-trans retinoid acid and Exendin-4 in NOD mice. Int j endocrinol 2014;2014:435481.
Cheng P, et al. PLGA-PNIPAM microspheres loaded with the gastrointestinal nutrient NaB ameliorate cardiac dysfunction by activating Sirt3 in acute myocardial infarction. Advanced Science. 2016;3:1600254.
Capurso NA, et al. Development of a nanoparticulate formulation of retinoic acid that suppresses Th17 cells and upregulates regulatory T cells. Self/nonself. 2010;1:335–40.
Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19:373–9.
Lewis JS, et al. Dual-sized microparticle system for generating suppressive dendritic cells prevents and reverses type 1 diabetes in the nonobese diabetic mouse model. ACS Biomater Sci Eng. 2019;5:2631–46.
Lewis JS, et al. A combination dual-sized microparticle system modulates dendritic cells and prevents type 1 diabetes in prediabetic NOD mice. Clinic immunol (Orlando, Fla.). 2015;160:90–102.
Eppler HB, Jewell CM. Biomaterials as tools to decode immunity. Adv Mater. 2020;32.
Kespohl M, et al. The microbial metabolite butyrate induces expression of Th1-associated factors in CD4+ T Cells. Front Immunol. 2017;8:1036.
Charlton B, Lafferty KJ. The Th1/Th2 balance in autoimmunity. Curr Opinion in Immunol. 1995;7.
Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Sem Immunopathol. 2019;41.
Funding
This work was supported in part by the Juvenile Diabetes Research Foundation (2-SRA-2016–319-S-B) and the National Institute of Health (R01 # EB026896 NIBIB).
Author information
Authors and Affiliations
Contributions
All authors contributed to manuscript preparation and experimental design. SC performed experiments and analyzed data.
Corresponding author
Ethics declarations
Ethics approval
All studies involving animals were approved and carried out under the supervision of the University of Maryland Institutional Animal Care and Use Committee (IACUC) in compliance with local, state, and federal guidelines.
Consent to participate
No applicable.
Consent for publication
No applicable.
Conflict of interest
CJ is an employee of the VA Maryland Health Care System. CJ has equity positions with Avidea Technologies and Cellth Systems, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Carey, S.T., Gammon, J.M. & Jewell, C.M. Biomaterial-enabled induction of pancreatic-specific regulatory T cells through distinct signal transduction pathways. Drug Deliv. and Transl. Res. 11, 2468–2481 (2021). https://doi.org/10.1007/s13346-021-01075-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01075-5