Abstract
In type 1 diabetes, insulin remains the mature therapeutic cornerstone; yet, the increasing number of individuals developing type 1 diabetes (predominantly children and adolescents) still face severe complications. Fortunately, our understanding of type 1 diabetes is continuously being refined, allowing for refocused development of novel prevention and management strategies. Hitherto, attempts based on immune suppression and modulation have been only partly successful in preventing the key pathophysiological feature in type 1 diabetes: the immune-mediated derangement or destruction of beta cells in the pancreatic islets of Langerhans, leading to low or absent insulin secretion and chronic hyperglycaemia. Evidence now warrants a focus on the beta cell itself and how to avoid its dysfunction, which is putatively caused by cytokine-driven inflammation and other stress factors, leading to low insulin-secretory capacity, autoantigen presentation and immune-mediated destruction. Correspondingly, beta cell rescue strategies are being pursued, which include antigen vaccination using, for example, oral insulin or peptides, as well as agents with suggested benefits on beta cell stress, such as verapamil and glucagon-like peptide-1 receptor agonists. Whilst autoimmune-focused prevention approaches are central in type 1 diabetes and will be a requirement in the advent of stem cell-based replacement therapies, managing the primarily cardiometabolic complications of established type 1 diabetes is equally essential. In this review, we outline selected recent and suggested future attempts to address the evolving profile of the person with type 1 diabetes.
Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to prolonging the life expectancy of people living with type 1 diabetes, the discovery of insulin a century ago revolutionised the management of this chronic autoimmune disease. Today, type 1 diabetes is the most common type of diabetes in children, and estimates suggest that around 100,000 children develop the disease every year [1]. Unfortunately, despite the availability of advanced insulins, affected individuals remain at high risk of serious complications, including cardiovascular mortality [2,3,4]. New interventions are, therefore, urgently required to improve the prognosis for the increasing number of people who are diagnosed with type 1 diabetes each year.
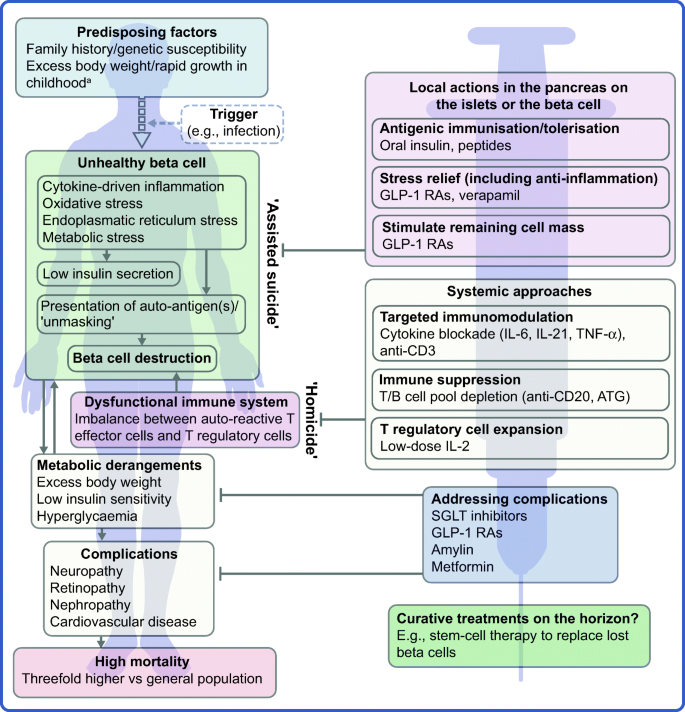
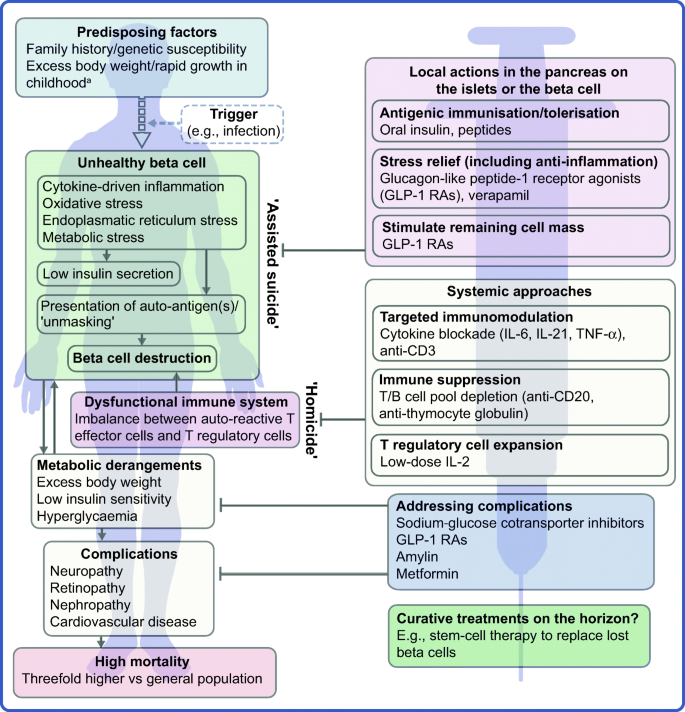
The profile of the person with type 1 diabetes is evolving and, with that, our understanding of the disease. The overall pathophysiological feature is loss of functional beta cell mass in the pancreatic islets of Langerhans (Fig. 1) [5]. Hypotheses suggest that the loss of functional beta cell mass occurs in a chain of events analogous to an ‘assisted suicide’ [6, 7], where the demise of the beta cell is likely due to a combination of a dysfunctional beta cell that becomes more visible to the immune system, which, in turn, overreacts and destroys the beta cell.
Hallmarks of the evolving profile of the individual with type 1 diabetes, and current and future options for the prevention of this disease and for the management of its associated complications. aAccording to some recent evidence [124,125,126,127,128,129,130]. This figure is available as a downloadable slide
In its early stage (Stage 1), type 1 diabetes is usually asymptomatic; however, the development of autoimmunity is often detectable in early life, with circulating autoantibodies targeting insulin or other proteins, such as GAD65, insulinoma-associated protein 2 (IA2) or zinc transporter 8 (ZNT8) [5]. When a large portion of the beta cell mass has become dysfunctional or lost, asymptomatic dysglycaemia (Stage 2) and, later, symptoms of hyperglycaemia (Stage 3) ensue due to insufficient or absent insulin secretion.
Type 1 diabetes is a polygenic disorder, in which susceptibility loci or genetic variation contributes to disease risk. The HLA region on chromosome 6 is the main susceptibility locus and, in recent years, many other loci across the genome have been associated with an increasing risk of the disease [8]. However, from studies in monozygotic twins, for whom the onset of type 1 diabetes can vary considerably [9], it has become evident that non-genetic factors play a major role in triggering or perpetuating overt type 1 diabetes. A multitude of efforts have failed at robustly identifying such factors, strongly indicating that no single pathogen is responsible. Viral infections have been suggested, including enteroviruses and human herpesvirus-6 [10,11,12,13]. Of note, however, studies (mainly in animals) have also suggested that several viral infections may prevent the development of type 1 diabetes [14, 15], in line with the ‘hygiene hypothesis’ [16, 17].
People living with type 1 diabetes remain dependent on exogenous insulins as the cornerstone therapeutic option [18]. Since the isolation of insulin in 1921, novel and versatile formulations, analogues and delivery vehicles have been introduced [19, 20]. Together with much improved glucose monitoring, these advances have contributed to the increases in the survival and life expectancy of individuals with type 1 diabetes [21]. Still, only a minority of people with type 1 diabetes achieve recommended glycaemic and time-in-range targets [22], and hyperglycaemia continues to be a risk factor for short-term metabolic and long-term macro- and microvascular complications [2, 23,24,25]. Further, the use of exogenous insulins requires unremitting glycaemic monitoring and dose titration to mitigate the risk of hypoglycaemia. The all-cause mortality risk is around threefold higher for the individual with type 1 diabetes than for the general population [2,3,4, 26], and type 1 diabetes has been shown to be linked to cardiovascular outcomes more than any other disease, including type 2 diabetes [2].
As mentioned earlier, novel interventions are needed for the prevention and management of type 1 diabetes. Whilst progress has been limited, the evolving profile of a person with type 1 diabetes suggests that beyond ensuring accurate titration of exogenous insulin, efficient management of the disease should rely on other additional principles. First, there is an obvious need to act early to prevent or delay the destruction of functional beta cell mass by immunomodulatory intervention or other disease-modifying means. Second, stimulating or reprogramming the remaining beta cell mass to secrete insulin in a balanced way is required to avoid major blood glucose excursions with the lowest possible exogenous insulin dose. Third, reducing the risk of long-term complications, such as cardiovascular and renal outcomes, seems increasingly important (Fig. 1). Below we review selected current and in-development interventions meeting these three criteria (Table 1).
Immune-focused therapies
The overarching goal of immune-focused therapies in type 1 diabetes is to prevent or delay the loss of functional beta cell mass. The traditional understanding of autoimmunity in type 1 diabetes has focused on systemic immune dysregulation and on autoreactive T cells that have evaded thymic selection and migrated to the periphery, where they destroy islets. This view on the pathogenesis of type 1 diabetes has been referred to as T cell-mediated ‘homicide’ [6]. Thus, recent efforts have concentrated on cell- or cytokine-directed interventions, which have been successful in other autoimmune diseases. Targeting T cells or proinflammatory cytokines remain valid efforts and many agents are in active development; so far, however, these approaches have been only partly successful. This arguably indicates a need to refocus hypotheses, as discussed later in this review (see ‘Future perspectives’ section), where we outline how the beta cell itself contributes to its own demise (the ‘assisted suicide’ hypothesis).
Cell-directed interventions
In line with the traditional immune-centric view on the pathogenesis of type 1 diabetes, many immunomodulatory strategies have focused on antibodies targeting T effector cells. The anti-CD3 antibodies teplizumab and otelixizumab have shown some attenuation of loss of beta cell function [27,28,29,30]. A Phase II trial with relatives with a high risk of developing type 1 diabetes indicated a more than 50% risk reduction with teplizumab (HR 0.41 vs placebo) and clinical type 1 diabetes diagnosis was delayed by 1.5–2 years [31]. Accordingly, teplizumab has recently been granted a breakthrough therapy status by the US Food and Drug Administration. An ongoing Phase III trial (PROTECT; ClinicalTrials.gov registration no. NCT03875729) aims to evaluate the benefits and safety of teplizumab in children and adolescents with recently diagnosed type 1 diabetes.
The presence of autoantibodies against beta cell antigens, such as GAD65 and insulin, has spurred attempts targeting B cell-related molecules. These efforts have been somewhat successful in animal models [32, 33], as well as clinically, most prominently with the B cell-depleting anti-CD20 antibody rituximab. Although rituximab led to detectable protraction of beta cell function [34], the effect was transient [35], exemplifying the fact that B cell-directed therapy alone does not appear to sustainably prevent or ameliorate beta cell autoimmunity. So far, however, B cell-directed agents have not been tested in the early disease stage, precluding conclusions regarding the usefulness of such interventions in delaying or even preventing progression to later stages.
In clinical investigations, low-dose anti-thymocyte globulin (ATG) treatment significantly (vs placebo) preserved C-peptide secretion and improved glycaemic control in children, as well as adults, with new-onset type 1 diabetes [36,37,38]. The potential benefits of ATG appear to depend on the dose level and the age of the recipients, and the clinical utility of the approach remains to be established. ATG in combination with granulocyte colony stimulating factor (GCSF) was also explored based on the hypothesis of a synergistic benefit of the combination of transient T cell depletion via low-dose ATG with the upregulation of activated T regulatory cells and tolerogenic dendritic cells induced by GCSF. However, the combination did not appear to offer a synergistic effect; in contrast to the use of ATG alone, ATG plus GCSF did not appear to be better than placebo in preserving C-peptide secretion [37].
Tissue-resident memory T effector cells, which likely play a role in many organ-specific autoimmune diseases, such as type 1 diabetes, are very difficult to eliminate. Alefacept, a T cell-depleting fusion protein that targets CD2 and, therefore, memory T effector cells, was tested in adolescents and young adults with Stage 3 type 1 diabetes in the T1DAL trial [39]. Although the trial did not complete enrolment as planned, it reported a trend for benefits with regard to beta cell preservation, reduced insulin requirements and low risk of hypoglycaemia that persisted throughout the follow-up of 15 months after treatment.
Importantly, whether considering the targeting of the T or B cell in type 1 diabetes, sufficient long-term benefits via systemic cell pool depletion comes with an inherent risk of introducing equally long-term or even irreversible changes to the immune system. Such changes may predispose the patient to a less favourable prognosis for chronic viral infections. For example, reactivation of Epstein-Barr virus (EBV) has been observed after anti-CD3 therapies [40, 41]. Mitigating such risks may be achieved using carefully tailored dosing regimens and monitoring; still, the seriousness of the risks may indicate an unfavourable benefit:risks balance. Therefore, non-depleting immunomodulation has been explored. For example, 24-month blockade of CD80 and CD86 via the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)-immunoglobulin fusion molecule abatacept markedly prolonged beta cell function in new-onset type 1 diabetes and was accompanied by increased numbers of naive T cells [42, 43].
Cytokine-directed interventions
Anti-inflammatory cytokine-specific compounds, which are successfully used, for example, in rheumatic diseases, have been tested as alternatives to directly targeting the T or B cell in type 1 diabetes, as briefly summarised below. In addition, to stimulate an increase in T regulatory cells, low-dose IL-2 treatment has also been tested and the results have been somewhat promising [44,45,46,47,48], with recent developments mitigating earlier caveats, which included an arguably narrow dose range and lack of full specificity for T regulatory cells.
Blockade or antagonism of the central proinflammatory cytokine TNF-α using infliximab, adalimumab or the receptor fusion protein etanercept have shown some potential in type 1 diabetes, with indications of improved glycaemic control and C-peptide secretion [49, 50]. More recently, a C-peptide-sparing effect of TNF-α blockade was reported with golimumab use, after 1 year in children and young adults with type 1 diabetes [51].
IL-6 is another proinflammatory cytokine that has been targeted with success in multiple other autoimmune diseases [52]. Although its role in type 1 diabetes is not established, IL-6 has been suggested as a target [53]. Of note, IL-6 has been shown to protect the beta cell from oxidative stress and is constitutively expressed by pancreatic alpha and beta cells, indicating important physiological roles [54]. In type 1 diabetes, the EXTEND Phase II trial of tocilizumab, a monoclonal antibody against the IL-6 receptor, was recently completed (ClinicalTrials.gov registration no. NCT02293837).
IL-21 has been proposed as an attractive target in type 1 diabetes [55, 56]. Physiologically, IL-21 is important not only for the function of T helper (Th) cells (Th17 and T follicular helper cells) but also for the generation and migration of CD8+ T cells. CD8+ T cells are now considered the chief T cell type accumulating in and around islets [57, 58] with pre-proinsulin emerging as a pivotal autoantigen driving their infiltration in type 1 diabetes [59]. IL-21 neutralisation has been shown to prevent diabetes in mice [60], and a C-peptide-sparing benefit of anti-IL-21 alone or in combination with the glucagon-like peptide-1 (GLP-1) receptor agonist (RA) liraglutide has been observed in a clinical proof-of-concept study [61], as described further below. Reassuringly, non-clinical models, including a viral type 1 diabetes model, showed a minor impact of IL-21 blockade on the immune repertoire [55].
Antigen vaccination
With the appeal of having no expected effect on acquired immunity, the overall aim of beta cell antigen vaccination is to induce tolerance by balancing the T cell population between auto-aggressive T effector cells and autoantigen-specific T regulatory cells. Induction of T regulatory cells carries the potential benefit of also downregulating the activity of proinflammatory antigen-presenting cells. The topic has been extensively reviewed in the past [62]. Briefly, inspired by successes with vaccination against, for example, peanut allergy, tolerisation of T effector cells has been attempted using administration of whole antigens, such as oral insulin, or of peptides. Whilst the concepts are promising and under active investigation, their effectiveness in humans is yet to be proven. For example, in at-risk children, oral insulin administration has previously failed to prevent type 1 diabetes [63, 64], speculatively due to a suboptimal dose level or unclear effects across risk-specific subgroups [65, 66], including those defined by insulin gene polymorphisms. Similar results and considerations have been reported for immunisation with GAD65 [67] and for peptide-based therapies [68, 69]. Further, the lack of full clarity regarding the mechanisms at play with antigen-based therapies outlines a number of shortcomings, including the fact that no biomarker is currently available to assist in establishing the optimal dose regimen.
Non-immunomodulatory adjunctives
We next focus on selected compounds that have gained attention due to their potential benefits as adjuncts to insulin in type 1 diabetes.
Amylin
Amylin deficiency is a recognised feature of type 1 diabetes [70]. As a neuroendocrine hormone, amylin inhibits glucagon secretion and contributes to reducing postprandial glucose variability. As an adjunct to meal-time insulin, the injectable amylin analogue pramlintide is approved only in the USA for the treatment of type 1 and type 2 diabetes alike [71]. In type 1 diabetes, pramlintide has been shown to improve postprandial glucose levels to some extent [72]. Its clinical use has been limited, arguably because of the modest efficacy alongside the occurrence of side effects, such as nausea and, most importantly, postprandial hypoglycaemia.
Metformin
Metformin is a low-cost agent with glucose-lowering effects that mainly occur via decreased hepatic glucose production. It is not a guideline-recommended option in type 1 diabetes. However, partly because of its ameliorating effect on insulin resistance, metformin has been somewhat promising in managing the disease, especially in children and adolescents, as well as in obese people with type 1 diabetes, with studies indicating reduced insulin requirements and body weight reduction [73,74,75]. In the large REducing With MetfOrmin Vascular Adverse Lesions (REMOVAL) trial, however, metformin did not reduce the long-term insulin needs or improve glycaemic control in people with long-standing type 1 diabetes and multiple cardiovascular risk factors [76].
Sodium-glucose cotransporter inhibitors
Sodium-glucose cotransporter (SGLT) inhibitors lower blood glucose levels by restraining the absorption of glucose in the small intestine and promoting the renal excretion of glucose [77]. Results with dapagliflozin, empagliflozin and sotagliflozin have indicated benefits of SGLT inhibition in managing type 1 diabetes when added to insulin [78,79,80,81,82,83]. Significant benefits included reduced insulin dose requirements, improved glycaemic control and reduced body weight [84]. So far, sotagliflozin and dapagliflozin are approved in Europe and Japan (but not the USA) as adjuncts to insulin for the management of overweight or obese people with type 1 diabetes when optimally titrated insulin alone does not provide adequate glycaemic control. Importantly, however, data suggest that the use of SGLT inhibitors in type 1 diabetes is associated with markedly increased risk of diabetic ketoacidosis [85,86,87]; for sotagliflozin, a 5–17-fold risk increase was noted [88]. These observations prompted the formation of an international consensus on recommendations for the use of SGLT inhibition in type 1 diabetes [89] as well as a suggestion that treatment should be overseen by specialists [88].
GLP-1 RAs
GLP-1 is a hormone of the incretin system that is secreted upon food intake. A marked uptake has been seen in the use of GLP-1 RAs in type 2 diabetes due to their pleiotropic glucose-dependent effects that improve glycaemic control and reduce body weight [90]. In contrast, GLP-1 agonism for the treatment of type 1 diabetes remains unproven, with initial results from smaller investigator-conceived studies being inconclusive. Recently, Phase II findings with the short-acting GLP-1 RA exenatide in adults with type 1 diabetes were negative. In two larger Phase III trials (ADJUNCT ONE and ADJUNCT TWO), the GLP-1 analogue liraglutide used as an adjunct to insulin appeared well-tolerated and improved HbA1c and reduced body weight [91, 92]. Both ADJUNCT trials indicated a minor increase in the risk of hypoglycaemia and hyperglycaemia with ketosis with liraglutide use, whereas the risk of diabetic ketoacidosis was negligible. Subsequently, a plethora of investigations have reached similar conclusions [93,94,95,96,97,98,99,100,101]. Nonetheless, the use of GLP-1 RAs in type 1 diabetes remains potentially useful, as discussed below.
Verapamil
Verapamil is a common calcium-channel blocker used for decades as an anti-hypertensive agent. In mouse models of type 1 diabetes, verapamil promoted survival of functional beta cells via a mechanism that involves reduced expression of the cellular redox regulator thioredoxin-interacting protein [102]. In a smaller Phase II trial, verapamil was better than placebo for preserving meal-stimulated C-peptide secretion in adults with type 1 diabetes and no safety concerns were identified [103]. Despite these findings, however, the place for verapamil as a disease-modifying agent in type 1 diabetes remains to be fully established.
Future perspectives
Although research into type 1 diabetes prevention and disease modification continues to produce encouraging data, none of the approaches discussed above appears sufficiently effective alone in preventing or managing type 1 diabetes. Future endeavours will, therefore, require a novel focus, leveraging prior experience with regard to the immunopathophysiology of type 1 diabetes, whilst also exploring the promise of combination therapies that integrate tried or new treatment modalities. In addition, lessons learned from type 2 diabetes with regard to the beneficial effects of certain agents on, for example, body weight and cardiorenal risk may also prove relevant in type 1 diabetes. We review selected future prospects addressing these aspects below.
Of further note, the lack of sufficient efficacy of previously tested therapies may also be related to the fact that type 1 diabetes is a heterogenous disease with diverse disease stages (Stages 1 to 3) and modifiers, such as age of onset or clinical diagnosis. Identifying the optimal timing of each type of intervention relative to the disease stages and the age of the patient is, therefore, important. For example, initiating an immunomodulatory intervention at Stage 1 (i.e. prior to clinical diagnosis) is not a straightforward decision and may be associated with clinical inertia. Moreover, an increased focus on disease endotypes (i.e. different biological processes under the type 1 diabetes umbrella) was recently suggested to ensure a precision-medicine approach to type 1 diabetes research and management [104].
Immune interventions
It is becoming increasingly clear that autoreactivity to islet antigens is also present in healthy individuals [59] and autoimmunity recurs after autologous nonmyeloablative haematopoietic stem cell transplantation [105, 106]. Thus, in line with the ‘assisted suicide’ theory introduced earlier [6, 7], it is also increasingly apparent that the development of type 1 diabetes does not only involve dysfunctional islets, but also beta cells that ‘unmask’ themselves to immune recognition and destruction. This notion supports two central realisations; first, it might explain why, in previous studies, immune therapy alone has failed to protect beta cell function over longer periods of time after onset of diabetes. Second, looking forward, novel type 1 diabetes therapies should pursue the holy grail of type 1 diabetes immune therapy: essentially agents that act locally in the islets, within the pancreas, either targeting the immune cells destroying the beta cell or the beta cell itself. Knowledge gained over the years regarding the beta cell has suggested multiple, yet putative reasons for the ‘unmasking’ of these cells. Potential reasons include the facts that beta cells are especially biosynthetically active and systemically exposed [107] and, therefore, susceptible to stress-induced production of autoantigenic proteins during, for example, infections [108,109,110]. Moreover, the beta cell might be vulnerable to both cytokine-mediated destruction [111] and various types of endoplasmic reticulum stress [112]. Relieving the beta cell of these burdens may provide an opportunity to save the beta cell without resorting to aggressive immune suppression.
With this in mind, we see the following two promising avenues as deserving increased focus going forward: (1) therapies aimed at inducing tolerance to beta cell antigens; and (2) the use of GLP-1 RAs that directly target the beta cells to enhance their function whilst also protecting them from immune-mediated inflammatory stress.
As discussed above, achieving antigenic tolerance has, so far, proven elusive but carries the crucial potential of leaving the overall capacity of the immune system intact whilst suppressing only the diabetogenic cell populations. Future studies need to establish whether inducing tolerance in humans can be achieved by clonal anergy or clonal deletion of effector cells, or whether antigen-specific regulatory cells may be able to suppress autoreactivity locally. Moreover, it needs to be clarified to what extent tissue-resident memory effector cells can be eliminated.
Recent evidence from rodent models indicates a role for GLP-1 RAs in protecting beta cells from apoptosis and in promoting beta cell replication and mass [113,114,115,116,117]. As such, although this remains to be confirmed, it is conceivable that GLP-1 RAs may offer a way to prevent the ‘unmasking’ of the beta cell to immune effector cells, for example, by downregulating expression of MHC class I proteins. Intriguingly, unpublished non-clinical evidence shows that liraglutide also limits immune cell infiltration into pseudo-islets (M. von Herrath, unpublished results). In addition, studies in NOD mice have shown that GLP-1 RAs administered in combination with various immunomodulatory agents, including anti-CD3 compounds [118], were more efficient in inducing diabetes remission than when given as monotherapy [119]. Furthermore, the anti-inflammatory effects of GLP-1 RAs are well-documented, with liraglutide being associated with reduced systemic levels of C-reactive protein and of proinflammatory cytokines, such as TNF-α, IL-1β and IL-6 [120,121,122,123]. Whilst these findings have mainly been observed in animal models or in type 2 diabetes, their relevance to (clinical) type 1 diabetes is conceivable but, so far, largely unexplored.
Management of cardiometabolic complications
A person diagnosed with type 1 diabetes faces a high risk of serious complications and of premature death, primarily for cardiovascular causes. This warrants a therapeutic focus on the broad pathophysiology of the disease.
Further, whilst the exact connections between excess body weight and type 1 diabetes remain debatable [124], the increased incidence of type 1 diabetes seems to coincide with the rapid rise in the prevalence of obesity [125, 126]. Recent evidence suggests that a high BMI may exacerbate the early-stage immune-mediated beta cell destruction in type 1 diabetes, especially in children and adolescents [127]. Evidence also points to an impact of rapid growth in early childhood [128], and a positive correlation between the age of type 1 diabetes onset and BMI has been observed [129]. The ‘accelerator hypothesis’ views high BMI and low insulin sensitivity as triggers for type 1 diabetes onset [130] and the term ‘double diabetes’ has been suggested to describe an amalgam of type 1 diabetes with parallel and separate pathophysiological processes typically associated with type 2 diabetes, such as obesity and insulin resistance [131].
Use of SGLT inhibitors or GLP-1 RAs as adjuncts to insulin admittedly holds promise in ameliorating multiple type 1 diabetes complications. For example, evidence suggests that SGLT inhibitors offer cardiorenal protection [132, 133], at least in type 2 diabetes, putatively owing to clinically unproven mechanisms of action beyond improved glucose homeostasis [134]. Moreover, a few GLP-1 RAs (dulaglutide, liraglutide and semaglutide) are now indicated to reduce cardiovascular risk in people with type 2 diabetes and established cardiovascular disease, and a protective effect of GLP-1 RAs on the kidneys is suggested from a range of cardiovascular outcome trials (CVOTs) in type 2 diabetes [135,136,137,138]. In addition, both SGLT inhibitors and GLP-1 RAs, especially second-generation GLP-1 RAs (e.g., semaglutide), are associated with a meaningful reducing effect on body weight.
Combination therapies
Combination therapies that work via two mechanistically distinct targets to integrate immune modulation with a beta cell-specific component have been suggested [139,140,141] and encouraged [142]. Truly advantageous combination therapies are arguably those in which the components target different pathogenic pathways (for example, systemic vs beta cell-specific pathways), thereby synergising in terms of the beneficial effects. These combination therapies should also be safe and well-tolerated alone and in combination.
Known ongoing efforts are sparse but include the combination of ATG and GCSF (as discussed above) and the combination of targeted immune modulation via an anti-IL-21 antibody in combination with a GLP-1 analogue (liraglutide). In addition to the potential of preserving functional beta cell mass by leveraging the immunomodulatory and anti-inflammatory properties of both the anti-IL-21 antibody and liraglutide, their combination addresses the need to manage the symptoms and complications of established type 1 diabetes, as discussed earlier. As previously mentioned, results from a clinical proof-of-concept trial recently found that anti-IL-21 plus liraglutide was significantly better than placebo in preserving C-peptide secretion over a period of 54 weeks [61]. The benefits diminished after treatment cessation; however, the treatment appeared safe and well-tolerated.
Stem cell replacement therapy
On the horizon, we approach the promise of stem cell-based therapies [143], offering a potential cure by replacing or supplementing beta cells that have been lost or have become dysfunctional. Stem cell-derived beta cells, however, also need to be rescued from immune-mediated destruction, suggesting that some degree of immunomodulation will be needed, even in the advent of viable stem cell therapy in type 1 diabetes, unless a fully effective immune-defying capsule is available [144]. In this context, better prevention or treatment regimens will also be useful for enabling longer-term beta cell graft acceptance.
Closing thoughts
Whilst many intriguing non-insulin therapies have failed to fully meet their potential in the past few decades, hope remains that the knowledge gained has carved out paths towards better options for the prevention and management of type 1 diabetes. Taken together, in our view, stem cell replacement therapies and a refocused development of safe and well-tolerated combination therapies are the most promising emerging preventive or therapeutic avenues. In parallel, reinforced efforts to predict or diagnose type 1 diabetes as soon as possible are equally important in light of the fact that even the best interventions need to be introduced as early as possible to effectively preserve or rescue beta cells in individuals with this condition.
Abbreviations
- ATG:
-
Anti-thymocyte globulin
- GCSF:
-
Granulocyte colony stimulating factor
- GLP-1:
-
Glucagon-like peptide-1
- RA:
-
Receptor agonist
- SGLT:
-
Sodium-glucose cotransporter
- Th:
-
T helper
References
Patterson CC, Karuranga S, Salpea P et al (2019) Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157:107842. https://doi.org/10.1016/j.diabres.2019.107842
Petrie JR, Sattar N (2019) Excess cardiovascular risk in type 1 diabetes mellitus. Circulation 139(6):744–747. https://doi.org/10.1161/circulationaha.118.038137
Schofield J, Ho J, Soran H (2019) Cardiovascular risk in type 1 diabetes mellitus. Diabetes Ther 10(3):773–789. https://doi.org/10.1007/s13300-019-0612-8
Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM (2006) High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care 29(4):798–804. https://doi.org/10.2337/diacare.29.04.06.dc05-1433
Katsarou A, Gudbjörnsdottir S, Rawshani A et al (2017) Type 1 diabetes mellitus. Nat Rev Dis Primers 3:17016. https://doi.org/10.1038/nrdp.2017.16
Atkinson MA, Bluestone JA, Eisenbarth GS et al (2011) How does type 1 diabetes develop?: the notion of homicide or β-cell suicide revisited. Diabetes 60(5):1370–1379. https://doi.org/10.2337/db10-1797
Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A (2020) Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat Rev Endocrinol 1–12. https://doi.org/10.1038/s41574-020-00443-4
Pociot F, Lernmark Å (2016) Genetic risk factors for type 1 diabetes. Lancet 387(10035):2331–2339. https://doi.org/10.1016/S0140-6736(16)30582-7
Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T (2008) Concordance for islet autoimmunity among monozygotic twins. N Engl J Med 359(26):2849–2850. https://doi.org/10.1056/NEJMc0805398
Sabouri S, Benkahla MA, Kiosses WB et al (2020) Human herpesvirus-6 is present at higher levels in the pancreatic tissues of donors with type 1 diabetes. J Autoimmun 107:102378. https://doi.org/10.1016/j.jaut.2019.102378
Rodriguez-Calvo T (2018) Enteroviral infections as a trigger for type 1 diabetes. Curr Diab Rep 18(11):106. https://doi.org/10.1007/s11892-018-1077-2
Richardson SJ, Morgan NG (2018) Enteroviral infections in the pathogenesis of type 1 diabetes: new insights for therapeutic intervention. Curr Opin Pharmacol 43:11–19. https://doi.org/10.1016/j.coph.2018.07.006
Rodriguez-Calvo T, Sabouri S, Anquetil F, von Herrath MG (2016) The viral paradigm in type 1 diabetes: who are the main suspects? Autoimmun Rev 15(10):964–969. https://doi.org/10.1016/j.autrev.2016.07.019
Filippi C, von Herrath M (2005) How viral infections affect the autoimmune process leading to type 1 diabetes. Cell Immunol 233(2):125–132. https://doi.org/10.1016/j.cellimm.2005.04.009
Christen U, von Herrath MG (2011) Do viral infections protect from or enhance type 1 diabetes and how can we tell the difference? Cell Mol Immunol 8(3):193–198. https://doi.org/10.1038/cmi.2010.71
Bach JF (2001) Protective role of infections and vaccinations on autoimmune diseases. J Autoimmun 16(3):347–353. https://doi.org/10.1006/jaut.2000.0478
von Mutius E (2007) Allergies, infections and the hygiene hypothesis--the epidemiological evidence. Immunobiology 212(6):433–439. https://doi.org/10.1016/j.imbio.2007.03.002
American Diabetes Association (2020) 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2020. Diabetes Care 43(Supplement 1):S98. https://doi.org/10.2337/dc20-S009
Beck RW, Bergenstal RM, Laffel LM, Pickup JC (2019) Advances in technology for management of type 1 diabetes. Lancet 394(10205):1265–1273. https://doi.org/10.1016/s0140-6736(19)31142-0
Beck RW, Bergenstal RM, Riddlesworth TD et al (2019) Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 42(3):400–405. https://doi.org/10.2337/dc18-1444
Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ (2012) Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes 61(11):2987–2992. https://doi.org/10.2337/db11-1625
Weinstock RS, Schütz-Fuhrmann I, Connor CG et al (2016) Type 1 diabetes in older adults: comparing treatments and chronic complications in the United States T1D Exchange and the German/Austrian DPV registries. Diabetes Res Clin Pract 122:28–37. https://doi.org/10.1016/j.diabres.2016.09.024
Bebu I, Braffett BH, Pop-Busui R, Orchard TJ, Nathan DM, Lachin JM (2017) The relationship of blood glucose with cardiovascular disease is mediated over time by traditional risk factors in type 1 diabetes: the DCCT/EDIC study. Diabetologia 60(10):2084–2091. https://doi.org/10.1007/s00125-017-4374-4
Rawshani A, Rawshani A, Franzén S et al (2017) Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 376(15):1407–1418. https://doi.org/10.1056/NEJMoa1608664
Rawshani A, Sattar N, Franzén S et al (2018) Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 392(10146):477–486. https://doi.org/10.1016/s0140-6736(18)31506-x
Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK (2015) Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care 38(2):316–322. https://doi.org/10.2337/dc14-0920
Hagopian W, Ferry RJ, Sherry N et al (2013) Teplizumab preserves C-peptide in recent-onset type 1 diabetes. Diabetes 62(11):3901. https://doi.org/10.2337/db13-0236
Herold KC, Gitelman SE, Ehlers MR et al (2013) Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 62(11):3766–3774. https://doi.org/10.2337/db13-0345
Sherry N, Hagopian W, Ludvigsson J et al (2011) Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet 378(9790):487–497. https://doi.org/10.1016/S0140-6736(11)60931-8
Aronson R, Gottlieb PA, Christiansen JS et al (2014) Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care 37(10):2746–2754. https://doi.org/10.2337/dc13-0327
Herold KC, Bundy BN, Long SA et al (2019) An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 381(7):603–613. https://doi.org/10.1056/NEJMoa1902226
Hu CY, Rodriguez-Pinto D, Du W et al (2007) Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest 117(12):3857–3867. https://doi.org/10.1172/jci32405
Xiu Y, Wong CP, Bouaziz JD et al (2008) B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J Immunol 180(5):2863–2875. https://doi.org/10.4049/jimmunol.180.5.2863
Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H et al (2009) Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 361(22):2143–2152. https://doi.org/10.1056/NEJMoa0904452
Pescovitz MD, Greenbaum CJ, Bundy B et al (2014) B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care 37(2):453–459. https://doi.org/10.2337/dc13-0626
Gitelman SE, Gottlieb PA, Rigby MR et al (2013) Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol 1(4):306–316. https://doi.org/10.1016/S2213-8587(13)70065-2
Haller MJ, Schatz DA, Skyler JS et al (2018) Low-dose anti-thymocyte globulin (ATG) preserves β-cell function and improves HbA(1c) in new-onset type 1 diabetes. Diabetes Care 41(9):1917–1925. https://doi.org/10.2337/dc18-0494
Haller MJ, Long SA, Blanchfield JL et al (2019) Low-dose anti-thymocyte globulin preserves C-peptide, reduces HbA1c, and increases regulatory to conventional T-cell ratios in new-onset type 1 diabetes: two-year clinical trial data. Diabetes 68(6):1267–1276. https://doi.org/10.2337/db19-0057
Rigby MR, Harris KM, Pinckney A et al (2015) Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest 125(8):3285–3296. https://doi.org/10.1172/JCI81722
Keymeulen B, Candon S, Fafi-Kremer S et al (2010) Transient Epstein-Barr virus reactivation in CD3 monoclonal antibody-treated patients. Blood 115(6):1145–1155. https://doi.org/10.1182/blood-2009-02-204875
Kroll JL, Beam C, Li S et al (2013) Reactivation of latent viruses in individuals receiving rituximab for new onset type 1 diabetes. J Clin Virol 57(2):115–119. https://doi.org/10.1016/j.jcv.2013.01.016
Orban T, Bundy B, Becker DJ et al (2014) Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care 37(4):1069–1075. https://doi.org/10.2337/dc13-0604
Orban T, Bundy B, Becker DJ et al (2011) Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 378(9789):412–419. https://doi.org/10.1016/s0140-6736(11)60886-6
Dwyer CJ, Ward NC, Pugliese A, Malek TR (2016) Promoting immune regulation in type 1 diabetes using low-dose interleukin-2. Curr Diab Rep 16(6):46–46. https://doi.org/10.1007/s11892-016-0739-1
Hartemann A, Bensimon G, Payan CA et al (2013) Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 1(4):295–305. https://doi.org/10.1016/s2213-8587(13)70113-x
Rosenzwajg M, Salet R, Lorenzon R et al (2020) Low-dose IL-2 in children with recently diagnosed type 1 diabetes: a phase I/II randomised, double-blind, placebo-controlled, dose-finding study. Diabetologia 63(9):1808–1821. https://doi.org/10.1007/s00125-020-05200-w
Todd JA, Evangelou M, Cutler AJ et al (2016) Regulatory T cell responses in participants with type 1 diabetes after a single dose of interleukin-2: a non-randomised, open label, adaptive dose-finding trial. PLoS Med 13(10):e1002139. https://doi.org/10.1371/journal.pmed.1002139
Khoryati L, Pham MN, Sherve M et al (2020) An IL-2 mutein engineered to promote expansion of regulatory T cells arrests ongoing autoimmunity in mice. Sci Immunol 5(50):eaba5264. https://doi.org/10.1126/sciimmunol.aba5264
Mastrandrea L, Yu J, Behrens T et al (2009) Etanercept treatment in children with new-onset type 1 diabetes. Diabetes Care 32(7):1244. https://doi.org/10.2337/dc09-0054
Timper K, Hruz P, Beglinger C, Donath MY (2013) Infliximab in the treatment of Crohn disease and type 1 diabetes. Diabetes Care 36(7):e90. https://doi.org/10.2337/dc13-0199
Quattrin T, Haller MJ, Steck A et al (2020) 3-LB: golimumab (GLM) preserves ß-cell function and reduces insulin use and hypoglycemia in children and young adults with recently diagnosed type 1 diabetes (T1D): the phase 2 T1GER study. Diabetes 69(Supplement 1):3-LB (Abstract). https://doi.org/10.2337/db20-3-LB
Kang S, Tanaka T, Narazaki M, Kishimoto T (2019) Targeting interleukin-6 signaling in clinic. Immunity 50(4):1007–1023. https://doi.org/10.1016/j.immuni.2019.03.026
Hundhausen C, Roth A, Whalen E et al (2016) Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Sci Transl Med 8(356):356ra119. https://doi.org/10.1126/scitranslmed.aad9943
Rajendran S, Anquetil F, Quesada-Masachs E et al (2020) IL-6 is present in beta and alpha cells in human pancreatic islets: expression is reduced in subjects with type 1 diabetes. Clin Immunol 211:108320. https://doi.org/10.1016/j.clim.2019.108320
Van Belle TL, Nierkens S, Arens R, von Herrath MG (2012) Interleukin-21 receptor-mediated signals control autoreactive T cell infiltration in pancreatic islets. Immunity 36(6):1060–1072. https://doi.org/10.1016/j.immuni.2012.04.005
Sutherland AP, Van Belle T, Wurster AL et al (2009) Interleukin-21 is required for the development of type 1 diabetes in NOD mice. Diabetes 58(5):1144–1155. https://doi.org/10.2337/db08-0882
Pugliese A (2017) Autoreactive T cells in type 1 diabetes. J Clin Invest 127(8):2881–2891. https://doi.org/10.1172/jci94549
Babon JA, DeNicola ME, Blodgett DM et al (2016) Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med 22(12):1482–1487. https://doi.org/10.1038/nm.4203
Bender C, Rodriguez-Calvo T, Amirian N, Coppieters KT, von Herrath MG (2020) The healthy exocrine pancreas contains preproinsulin-specific CD8 T cells that attack islets in type 1 diabetes. Sci Adv 6(42):eabc5586. https://doi.org/10.1126/sciadv.abc5586
McGuire HM, Walters S, Vogelzang A et al (2011) Interleukin-21 is critically required in autoimmune and allogeneic responses to islet tissue in murine models. Diabetes 60(3):867–875. https://doi.org/10.2337/db10-1157
Mathieu C, von Herrath M, Bain SC et al (2020) OP08-48: efficacy and safety of anti-interleukin (IL)-21 in combination with liraglutide in adults recently diagnosed with type 1 diabetes. Diabetologia 63(1):1–485 (Abstract). https://doi.org/10.1007/s00125-020-05221-5
Roep BO, Wheeler DCS, Peakman M (2019) Antigen-based immune modulation therapy for type 1 diabetes: the era of precision medicine. Lancet Diabetes Endocrinol 7(1):65–74. https://doi.org/10.1016/S2213-8587(18)30109-8
Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ (2017) Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA 318(19):1891–1902. https://doi.org/10.1001/jama.2017.17070
Skyler JS, Krischer JP, Wolfsdorf J et al (2005) Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial--Type 1. Diabetes Care 28(5):1068–1076. https://doi.org/10.2337/diacare.28.5.1068
Bonifacio E, Ziegler A-G, Klingensmith G et al (2015) Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT Randomized Clinical Trial. JAMA 313(15):1541–1549. https://doi.org/10.1001/jama.2015.2928
Ziegler AG, Achenbach P, Berner R et al (2019) Oral insulin therapy for primary prevention of type 1 diabetes in infants with high genetic risk: the GPPAD-POInT (global platform for the prevention of autoimmune diabetes primary oral insulin trial) study protocol. BMJ Open 9(6):e028578. https://doi.org/10.1136/bmjopen-2018-028578
Ludvigsson J, Wahlberg J, Casas R (2017) Intralymphatic injection of autoantigen in type 1 diabetes. N Engl J Med 376(7):697–699. https://doi.org/10.1056/NEJMc1616343
Bovy N, Boitard C, Achenbach P et al (2020) OP09-52: long-term follow-up study of type 1 diabetes patients previously treated with IMCY-0098 or placebo in young adults with recent-onset type 1 diabetes. Diabetologia 63(1):1–485 (Abstract). https://doi.org/10.1007/s00125-020-05221-5
Smith EL, Peakman M (2018) Peptide immunotherapy for type 1 diabetes-clinical advances. Front Immunol 9:392. https://doi.org/10.3389/fimmu.2018.00392
Martin C (2006) The physiology of amylin and insulin: maintaining the balance between glucose secretion and glucose uptake. Diabetes Educ 32(Suppl 3):101s–104s. https://doi.org/10.1177/0145721706288237
Ryan GJ, Jobe LJ, Martin R (2005) Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Clin Ther 27(10):1500–1512. https://doi.org/10.1016/j.clinthera.2005.10.009
Riddle MC, Nahra R, Han J et al (2018) Control of postprandial hyperglycemia in type 1 diabetes by 24-hour fixed-dose coadministration of pramlintide and regular human insulin: a randomized, two-way crossover study. Diabetes Care 41(11):2346–2352. https://doi.org/10.2337/dc18-1091
Lund SS, Tarnow L, Astrup AS et al (2008) Effect of adjunct metformin treatment in patients with type-1 diabetes and persistent inadequate glycaemic control. A randomized study. PLoS One 3(10):e3363. https://doi.org/10.1371/journal.pone.0003363
Libman IM, Miller KM, DiMeglio LA et al (2015) Effect of metformin added to insulin on glycemic control among overweight/obese adolescents with type 1 diabetes: a randomized clinical trial. JAMA 314(21):2241–2250. https://doi.org/10.1001/jama.2015.16174
Vella S, Buetow L, Royle P, Livingstone S, Colhoun HM, Petrie JR (2010) The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia 53(5):809–820. https://doi.org/10.1007/s00125-009-1636-9
Petrie JR, Chaturvedi N, Ford I et al (2017) Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 5(8):597–609. https://doi.org/10.1016/s2213-8587(17)30194-8
Ferrannini E (2017) Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab 26(1):27–38. https://doi.org/10.1016/j.cmet.2017.04.011
Buse JB, Garg SK, Rosenstock J et al (2018) Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 study. Diabetes Care 41(9):1970–1980. https://doi.org/10.2337/dc18-0343
Dandona P, Mathieu C, Phillip M et al (2018) Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care 41(12):2552–2559. https://doi.org/10.2337/dc18-1087
Mathieu C, Dandona P, Gillard P et al (2018) Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care 41(9):1938–1946. https://doi.org/10.2337/dc18-0623
Rosenstock J, Marquard J, Laffel LM et al (2018) Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 41(12):2560–2569. https://doi.org/10.2337/dc18-1749
Garg SK, Henry RR, Banks P et al (2017) Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med 377(24):2337–2348. https://doi.org/10.1056/NEJMoa1708337
Henry RR, Thakkar P, Tong C, Polidori D, Alba M (2015) Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care 38(12):2258–2265. https://doi.org/10.2337/dc15-1730
Tandon S, Ayis S, Hopkins D, Harding S, Stadler M (2021) The impact of pharmacological and lifestyle interventions on body weight in people with type 1 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 23(2):350–362. https://doi.org/10.1111/dom.14221
Taylor SI, Blau JE, Rother KI (2015) SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 100(8):2849–2852. https://doi.org/10.1210/jc.2015-1884
Taylor SI, Blau JE, Rother KI, Beitelshees AL (2019) SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. Lancet Diabetes Endocrinol 7(12):949–958. https://doi.org/10.1016/S2213-8587(19)30154-8
Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB (2015) Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 38(9):1687–1693. https://doi.org/10.2337/dc15-0843
Wolfsdorf JI, Ratner RE (2019) SGLT inhibitors for type 1 diabetes: proceed with extreme caution. Diabetes Care 42(6):991. https://doi.org/10.2337/dci19-0008
Danne T, Garg S, Peters AL et al (2019) International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care 42(6):1147–1154. https://doi.org/10.2337/dc18-2316
Aroda VR (2018) A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab 20(Suppl 1):22–33. https://doi.org/10.1111/dom.13162
Mathieu C, Zinman B, Hemmingsson JU et al (2016) Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE treat-to-target randomized trial. Diabetes Care 39(10):1702–1710. https://doi.org/10.2337/dc16-0691
Ahrén B, Hirsch IB, Pieber TR et al (2016) Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial. Diabetes Care 39(10):1693–1701. https://doi.org/10.2337/dc16-0690
Dimitrios P, Michael D, Vasilios K et al (2020) Liraglutide as adjunct to insulin treatment in patients with type 1 diabetes: a systematic review and meta-analysis. Curr Diabetes Rev 16(4):313–326. https://doi.org/10.2174/1573399815666190614141918
Ghanim H, Batra M, Green K et al (2020) Liraglutide treatment in overweight and obese patients with type 1 diabetes: a 26-week randomized controlled trial; mechanisms of weight loss. Diabetes Obes Metab 22(10):1742–1752. https://doi.org/10.1111/dom.14090
Goyal I, Sattar A, Johnson M, Dandona P (2020) Adjunct therapies in treatment of type 1 diabetes. J Diabetes 12(10):742–753. https://doi.org/10.1111/1753-0407.13078
Kuhadiya ND, Prohaska B, Ghanim H, Dandona P (2019) Addition of glucagon-like peptide-1 receptor agonist therapy to insulin in C-peptide-positive patients with type 1 diabetes. Diabetes Obes Metab 21(4):1054–1057. https://doi.org/10.1111/dom.13609
Wang W, Liu H, Xiao S, Liu S, Li X, Yu P (2017) Effects of insulin plus glucagon-like peptide-1 receptor agonists (GLP-1RAs) in treating type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther 8(4):727–738. https://doi.org/10.1007/s13300-017-0282-3
Dejgaard TF, Schmidt S, Frandsen CS et al (2020) Liraglutide reduces hyperglycaemia and body weight in overweight, dysregulated insulin-pump-treated patients with type 1 diabetes: the Lira Pump trial—a randomized, double-blinded, placebo-controlled trial. Diabetes Obes Metab 22(4):492–500. https://doi.org/10.1111/dom.13911
Dejgaard TF, Frandsen CS, Hansen TS et al (2016) Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 4(3):221–232. https://doi.org/10.1016/s2213-8587(15)00436-2
Frandsen CS, Dejgaard TF, Holst JJ, Andersen HU, Thorsteinsson B, Madsbad S (2015) Twelve-week treatment with liraglutide as add-on to insulin in normal-weight patients with poorly controlled type 1 diabetes: a randomized, placebo-controlled, double-blind parallel study. Diabetes Care 38(12):2250–2257. https://doi.org/10.2337/dc15-1037
Johansen NJ, Dejgaard TF, Lund A et al (2020) Efficacy and safety of meal-time administration of short-acting exenatide for glycaemic control in type 1 diabetes (MAG1C): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 8(4):313–324. https://doi.org/10.1016/s2213-8587(20)30030-9
Xu G, Chen J, Jing G, Shalev A (2012) Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes 61(4):848–856. https://doi.org/10.2337/db11-0955
Ovalle F, Grimes T, Xu G et al (2018) Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat Med 24(8):1108–1112. https://doi.org/10.1038/s41591-018-0089-4
Battaglia M, Ahmed S, Anderson MS et al (2020) Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 43(1):5–12. https://doi.org/10.2337/dc19-0880
Voltarelli JC, Couri CE, Stracieri AB et al (2007) Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 297(14):1568–1576. https://doi.org/10.1001/jama.297.14.1568
Voltarelli JC, Couri CE, Stracieri AB et al (2008) Autologous hematopoietic stem cell transplantation for type 1 diabetes. Ann N Y Acad Sci 1150:220–229. https://doi.org/10.1196/annals.1447.048
Mallone R, Eizirik DL (2020) Presumption of innocence for beta cells: why are they vulnerable autoimmune targets in type 1 diabetes? Diabetologia 63(10):1999–2006. https://doi.org/10.1007/s00125-020-05176-7
James EA, Pietropaolo M, Mamula MJ (2018) Immune recognition of β-cells: neoepitopes as key players in the loss of tolerance. Diabetes 67(6):1035–1042. https://doi.org/10.2337/dbi17-0030
Mauvais FX, Diana J, van Endert P (2016) Beta cell antigens in type 1 diabetes: triggers in pathogenesis and therapeutic targets. F1000Research 5:F1000 Faculty Rev-1728. https://doi.org/10.12688/f1000research.7411.1
Itariu BK, Stulnig TM (2014) Autoimmune aspects of type 2 diabetes mellitus - a mini-review. Gerontology 60(3):189–196. https://doi.org/10.1159/000356747
Burrack AL, Martinov T, Fife BT (2017) T cell-mediated beta cell destruction: autoimmunity and alloimmunity in the context of type 1 diabetes. Front Endocrinol 8:343. https://doi.org/10.3389/fendo.2017.00343
Marré ML, James EA, Piganelli JD (2015) β cell ER stress and the implications for immunogenicity in type 1 diabetes. Front Cell Dev Biol 3:67. https://doi.org/10.3389/fcell.2015.00067
Linnemann AK, Neuman JC, Battiola TJ, Wisinski JA, Kimple ME, Davis DB (2015) Glucagon-like peptide-1 regulates cholecystokinin production in β-cells to protect from apoptosis. Mol Endocrinol 29(7):978–987. https://doi.org/10.1210/me.2015-1030
Lee YS, Jun HS (2014) Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metab Clin Exp 63(1):9–19. https://doi.org/10.1016/j.metabol.2013.09.010
Villalba A, Rodriguez-Fernandez S, Perna-Barrull D et al (2020) Repurposed analog of GLP-1 ameliorates hyperglycemia in type 1 diabetic mice through pancreatic cell reprogramming. Front Endocrinol 11:258
Rowlands J, Heng J, Newsholme P, Carlessi R (2018) Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front Endocrinol 9:672–672. https://doi.org/10.3389/fendo.2018.00672
Buteau J, Foisy S, Joly E, Prentki M (2003) Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 52(1):124–132. https://doi.org/10.2337/diabetes.52.1.124
Sherry NA, Chen W, Kushner JA et al (2007) Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology 148(11):5136–5144. https://doi.org/10.1210/en.2007-0358
Rother KI, Spain LM, Wesley RA et al (2009) Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care 32(12):2251–2257. https://doi.org/10.2337/dc09-0773
Hogan AE, Gaoatswe G, Lynch L et al (2014) Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia 57(4):781–784. https://doi.org/10.1007/s00125-013-3145-0
von Scholten BJ, Persson F, Rosenlund S et al (2017) Effects of liraglutide on cardiovascular risk biomarkers in patients with type 2 diabetes and albuminuria: a sub-analysis of a randomized, placebo-controlled, double-blind, crossover trial. Diabetes Obes Metab 19(6):901–905. https://doi.org/10.1111/dom.12884
Pi-Sunyer X, Astrup A, Fujioka K et al (2015) A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 373(1):11–22. https://doi.org/10.1056/NEJMoa1411892
Bouchi R, Nakano Y, Fukuda T et al (2017) Reduction of visceral fat by liraglutide is associated with ameliorations of hepatic steatosis, albuminuria, and micro-inflammation in type 2 diabetic patients with insulin treatment: a randomized control trial. Endocr J 64(3):269–281. https://doi.org/10.1507/endocrj.EJ16-0449
Chobot A, Górowska-Kowolik K, Sokołowska M, Jarosz-Chobot P (2018) Obesity and diabetes-not only a simple link between two epidemics. Diabetes Metab Res Rev 34(7):e3042. https://doi.org/10.1002/dmrr.3042
Corbin KD, Driscoll KA, Pratley RE, Smith SR, Maahs DM, Mayer-Davis EJ (2018) Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev 39(5):629–663. https://doi.org/10.1210/er.2017-00191
Liu LL, Lawrence JM, Davis C et al (2010) Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes 11(1):4–11. https://doi.org/10.1111/j.1399-5448.2009.00519.x
Ferrara-Cook C, Geyer SM, Evans-Molina C et al (2020) Excess BMI accelerates islet autoimmunity in older children and adolescents. Diabetes Care 43(3):580–587. https://doi.org/10.2337/dc19-1167
The EURODIAB Substudy 2 Study Group (2002) Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care 25(10):1755–1760. https://doi.org/10.2337/diacare.25.10.1755
Giménez M, Aguilera E, Castell C, de Lara N, Nicolau J, Conget I (2007) Relationship between BMI and age at diagnosis of type 1 diabetes in a Mediterranean area in the period of 1990–2004. Diabetes Care 30(6):1593. https://doi.org/10.2337/dc06-2578
Wilkin TJ (2009) The accelerator hypothesis: a review of the evidence for insulin resistance as the basis for type I as well as type II diabetes. Int J Obes 33(7):716–726. https://doi.org/10.1038/ijo.2009.97
Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR (2013) Insulin resistance in type 1 diabetes: what is ‘double diabetes’ and what are the risks? Diabetologia 56(7):1462–1470. https://doi.org/10.1007/s00125-013-2904-2
Zelniker TA, Wiviott SD, Raz I et al (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393(10166):31–39. https://doi.org/10.1016/S0140-6736(18)32590-X
Neuen BL, Young T, Heerspink HJL et al (2019) SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 7(11):845–854. https://doi.org/10.1016/s2213-8587(19)30256-6
Packer M (2020) SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care 43:508–511. https://doi.org/10.2337/dci19-0074
Mann JFE, Ørsted DD, Brown-Frandsen K et al (2017) Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 377(9):839–848. https://doi.org/10.1056/NEJMoa1616011
Leiter LA, Bain SC, Bhatt DL et al (2020) The effect of glucagon-like peptide-1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across baseline blood pressure categories: analysis of the LEADER and SUSTAIN 6 trials. Diabetes Obes Metab 22(9):1690–1695. https://doi.org/10.1111/dom.14079
Gerstein HC, Colhoun HM, Dagenais GR et al (2019) Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394(10193):121–130. https://doi.org/10.1016/S0140-6736(19)31149-3
Tuttle KR, Rayner BL, Lakshmanan M et al (2019) 233-OR: chronic kidney disease (CKD) outcomes with dulaglutide (DU) vs. insulin glargine (IG) in type 2 diabetes (T2D) and moderate-to-severe CKD by albuminuria status: AWARD-7. Diabetes 68(Suppl 1):233-OR (Abstract). https://doi.org/10.2337/db19-233-OR
Kolb H, von Herrath M (2017) Immunotherapy for type 1 diabetes: why do current protocols not halt the underlying disease process? Cell Metab 25(2):233–241. https://doi.org/10.1016/j.cmet.2016.10.009
Bone RN, Evans-Molina C (2017) Combination immunotherapy for type 1 diabetes. Curr Diab Rep 17(7):50. https://doi.org/10.1007/s11892-017-0878-z
von Herrath M, Peakman M, Roep B (2013) Progress in immune-based therapies for type 1 diabetes. Clin Exp Immunol 172(2):186–202. https://doi.org/10.1111/cei.12085
Atkinson MA, Roep BO, Posgai A, Wheeler DCS, Peakman M (2019) The challenge of modulating β-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol 7(1):52–64. https://doi.org/10.1016/s2213-8587(18)30112-8
Chen S, Du K, Zou C (2020) Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Res Ther 11(1):275. https://doi.org/10.1186/s13287-020-01793-6
Coppieters K, Winkel L, von Herrath M (2020) Long-term viability through selective permeability. Nat Biomed Eng 4(8):763–764. https://doi.org/10.1038/s41551-020-0602-1
Ratner RE, Dickey R, Fineman M et al (2004) Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med 21(11):1204–1212. https://doi.org/10.1111/j.1464-5491.2004.01319.x
Whitehouse F, Kruger DF, Fineman M et al (2002) A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care 25(4):724–730. https://doi.org/10.2337/diacare.25.4.724
Authors’ relationships and activities
All authors are employees of Novo Nordisk A/S, Denmark.
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to the preparation of the review and approved the version to be published.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Figure slide
(PPTX 230 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
von Scholten, B.J., Kreiner, F.F., Gough, S.C.L. et al. Current and future therapies for type 1 diabetes. Diabetologia 64, 1037–1048 (2021). https://doi.org/10.1007/s00125-021-05398-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-021-05398-3