Abstract
Purpose
This study aims at understanding how the acidogenic fermentation microbial community was impacted by the hydrodynamic cavitation (HC) pre-treatment of the substrates’ mixture, constituted by waste-activated sludge and vegetable waste 1:1 on a TVS basis.
Methods
HC was performed with power = 8 kW, P = 1.4–1.5 bar, Qmixture of 25–30 L/min, 1550–1650 rpm, duration: 30 min. Fermentation tests were conducted on cavitated (CAV) and not cavitated (NCAV) mixture at T = 37 °C inside 4 L reactors in batch mode, then switched to semi-continuous with an OLR of 8 kgTVS m−3 d−1. Microbial community was characterized by 16S rRNA sequencing at the beginning and end of the pseudo-steady-state. Ecological diversity and clustering among the samples were determined by beta diversity, Venn diagram, and non-metric multi-dimensional scaling (NMDS) analysis.
Results
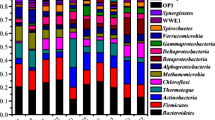
Cavitation was efficient in substrates’ hydrolyzation but resulted in a lower microbial diversity of 3.85 (Shannon Index) and VFAs concentration of 12.9 gCODVFA L−1 in the anaerobically fermented cavitated mixture (AF-CAV), respect to 4.54 and 18.2 gCODVFA L−1 in the anaerobically fermented not cavitated mixture (AF-NCAV), respectively. NMDS analysis showed that AF-CAV and AF-NCAV samples formed two different clusters, with VFAs concentration as the only significant factor explaining their difference (R2 = 1, Pr > r = 0.04167). Functional redundancy among community members probably allowed to maintain a stable VFAs composition despite the microbial community variation observed at the end of the test.
Conclusion
The insights here provided on the effects of HC confirm the fundamental role played by microbial community in acidogenic fermentation processes and underline its importance in evaluating the effect of substrates’ pre-treatment.
Graphical Abstract





Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- HC:
-
Hydrodynamic cavitation
- HRT:
-
Hydraulic retention time
- OLR:
-
Organic loading rate
- TS:
-
Total solids
- TVS:
-
Total volatile solids
- UC:
-
Ultrasound cavitation
- VFAs:
-
Volatile fatty acids
- WAS:
-
Waste-activated sludge
- NMDS:
-
Non-metric multi-dimensional scaling
References
Global Footprint Network: Earth overshoot day (2023). https://www.overshootday.org/
Costanza, R., Fioramonti, L., Kubiszewski, I.: The UN sustainable development goals and the dynamics of well-being. Front. Ecol. Environ. 14, 59 (2016)
Akinsemolu, A.A.: The role of microorganisms in achieving the sustainable development goals. J. Clean. Prod. 182, 139–155 (2018)
Ramos-Suarez, M., Zhang, Y., Outram, V.: Current perspectives on acidogenic fermentation to produce volatile fatty acids from waste. Rev. Environ. Sci. Biotechnol. 20, 439–478 (2021)
Atasoy, M., Owusu-Agyeman, I., Plaza, E., Cetecioglu, Z.: Bio-based volatile fatty acid production and recovery from waste streams: current status and future challenges. Bioresour. Technol. 268, 773–786 (2018)
Lytras, G., Lytras, C., Mathioudakis, D., Papadopoulou, K., Lyberatos, G.: Food waste valorization based on anaerobic digestion. Waste Biomass Val. 12, 1677–1697 (2021)
Battista, F., Frison, N., Pavan, P., et al.: Food wastes and sewage sludge as feedstock for an urban biorefinery producing biofuels and added-value bioproducts. J. Chem. Technol. Biotechnol. 95, 328–338 (2020)
Francini, G., Lombardi, L., Freire, F., Pecorini, I., Marques, P.: Environmental and cost life cycle analysis of different recovery processes of organic fraction of municipal solid waste and sewage sludge. Waste Biomass Val. 10, 3613–3634 (2019)
Vidal-Antich, C., Perez-Esteban, N., Astals, S., Peces, M., Mata-Alvarez, J., Dosta, J.: Assessing the potential of waste activated sludge and food waste co-fermentation for carboxylic acids production. Sci. Total Environ. 757, 143763 (2021)
Fang, W., Zhang, X., Zhang, P., Wan, J., Guo, H., Ghasimi, D.S.M., Morera, X.C., Zhang, T.: Overview of key operation factors and strategies for improving fermentative volatile fatty acid production and product regulation from sewage sludge. J. Environ. Sci. 87, 93–111 (2020)
Tonanzi, B., Gallipoli, A., Annesini, M.C., La, P.C., Gianico, A., Braguglia, C.M.: Pre-treatments and anaerobic hydrolysis as strategical key steps for resource recovery from sludge: the role of disintegration degree in metals leaching. J. Environ. Chem. Eng. (2021). https://doi.org/10.1016/j.jece.2020.104649
Bhat, A.P., Gogate, P.R.: Cavitation-based pre-treatment of wastewater and waste sludge for improvement in the performance of biological processes: a review. J. Environ. Chem. Eng. 9, 104743 (2021)
Carpenter, J., Badve, M., Rajoriya, S., George, S., Saharan, V.K., Pandit, A.B.: Hydrodynamic cavitation: an emerging technology for the intensification of various chemical and physical processes in a chemical process industry. Rev. Chem. Eng. 33, 433–468 (2017)
Dauptain, K., Trably, E., Santa-Catalina, G., Bernet, N., Carrere, H.: Role of indigenous bacteria in dark fermentation of organic substrates. Bioresour. Technol. 313, 123665 (2020)
Liu, N., Jiang, J., Yan, F., Gao, Y., Meng, Y., Aihemaiti, A., Ju, T.: Enhancement of volatile fatty acid production and biogas yield from food waste following sonication pretreatment. J. Environ. Manag. 217, 797–804 (2018)
Yang, G., Wang, J.: Biohydrogen production from waste activated sludge pretreated by combining sodium citrate with ultrasonic: energy conversion and microbial community. Energy Convers. Manag. 225, 113436 (2020)
Cesaro, A., Naddeo, V., Amodio, V., Belgiorno, V.: Enhanced biogas production from anaerobic codigestion of solid waste by sonolysis. Ultrason Sonochem 19, 596–600 (2012)
Lanfranchi, A., Tassinato, G., Valentino, F., Martinez, G.A., Jones, E., Gioia, C., Bertin, L., Cavinato, C.: Hydrodynamic cavitation pre-treatment of urban waste: integration with acidogenic fermentation, PHAs synthesis and anaerobic digestion processes. Chemosphere 301, 134624 (2022)
Cabrol, L., Marone, A., Tapia-Venegas, E., Steyer, J.-P., Ruiz-Filippi, G., Trably, E.: Microbial ecology of fermentative hydrogen producing bioprocesses: useful insights for driving the ecosystem function. FEMS Microbiol. Rev. 043, 158–181 (2017)
Luo, L., Sriram, S., Johnravindar, D., Louis Philippe Martin, T., Wong, J.W.C., Pradhan, N.: Effect of inoculum pretreatment on the microbial and metabolic dynamics of food waste dark fermentation. Bioresour. Technol. 358, 127404 (2022)
Moretto, G., Valentino, F., Pavan, P., Majone, M., Bolzonella, D.: Optimization of urban waste fermentation for volatile fatty acids production. Waste Manag. 92, 21–29 (2019)
Strazzera, G., Battista, F., Tonanzi, B., Rossetti, S., Bolzonella, D.: Optimization of short chain volatile fatty acids production from household food waste for biorefinery applications. Environ. Technol. Innov. 23, 101562 (2021)
Greses, S., Tomás-Pejó, E., Gónzalez-Fernández, C.: Agroindustrial waste as a resource for volatile fatty acids production via anaerobic fermentation. Bioresour. Technol. 297, 122486 (2020)
González-Fernández, C., García-Encina, P.A.: Impact of substrate to inoculum ratio in anaerobic digestion of swine slurry. Biomass Bioenerg. 33, 1065–1069 (2009)
Parada, A.E., Needham, D.M., Fuhrman, J.A.: Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 18, 1403–1414 (2016)
Apprill, A., Mcnally, S., Parsons, R., Weber, L.: Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 75, 129–137 (2015)
Bolyen, E., Rideout, J.R., Dillon, M.R., et al.: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019)
Chao, A.: Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 11, 265–270 (1984)
Shannon, C.E.: A mathematical theory of communication. Bell Syst. Tech. J. XXVII, 382–423 (1948)
Pielou, E.C.: Ecological Diversity. Wiley, New York (1975)
APAT-IRSA/CNR: Metodologie analitiche per il controllo della qualità delle acque. Poligrafica e Zecca dello Stato Roma (2003)
APHA/AWWA/WEF: Standard Methods for the Examination of Water and Wastewater, 23rd ed. (2017)
Wu, Q.L., Guo, W.Q., Zheng, H.S., Luo, H.C., Feng, X.C., Yin, R.L., Ren, N.Q.: Enhancement of volatile fatty acid production by co-fermentation of food waste and excess sludge without pH control: the mechanism and microbial community analyses. Bioresour. Technol. 216, 653–660 (2016)
Strazzera, G., Battista, F., Andreolli, M., Menini, M., Bolzonella, D., Lampis, S.: Influence of different household food wastes fractions on volatile fatty acids production by anaerobic fermentation. Bioresour. Technol. 335, 125289 (2021)
Llamas, M., Magdalena, J.A., Greses, S., Tomás-Pejó, E., González-Fernández, C.: Insights on the microbial communities developed during the anaerobic fermentation of raw and pretreated microalgae biomass. Chemosphere (2021). https://doi.org/10.1016/j.chemosphere.2020.127942
Le, N.T., Julcour-Lebigue, C., Delmas, H.: An executive review of sludge pretreatment by sonication. J. Environ. Sci. (China) 37, 139–153 (2015)
Lehne, G., Müller, A., Schwedes, J.: Mechanical disintegration of sewage sludge. Water Sci. Technol. 43, 19–26 (2001)
Chu, C.P., Chang, B.V., Liao, G.S., Jean, D.S., Lee, D.J.: Observations on changes in ultrasonically treated waste-activated sludge. Water Res. 35, 1038–1046 (2001)
Zhang, P., Zhang, G., Wang, W.: Ultrasonic treatment of biological sludge: floc disintegration, cell lysis and inactivation. Bioresour. Technol. 98, 207–210 (2007)
Zhang, L., Loh, K.C., Dai, Y., Tong, Y.W.: Acidogenic fermentation of food waste for production of volatile fatty acids: bacterial community analysis and semi-continuous operation. Waste Manag. 109, 75–84 (2020)
Yin, J., Yu, X., Zhang, Y., Shen, D., Wang, M., Long, Y., Chen, T.: Enhancement of acidogenic fermentation for volatile fatty acid production from food waste: effect of redox potential and inoculum. Bioresour. Technol. 216, 996–1003 (2016)
Iglesias-Iglesias, R., Campanaro, S., Treu, L., Kennes, C., Veiga, M.C.: Valorization of sewage sludge for volatile fatty acids production and role of microbiome on acidogenic fermentation. Bioresour. Technol. 291, 121817 (2019)
Gao, X., Zhang, Q., Zhu, H.: High rejection rate of polysaccharides by microfiltration benefits Christensenella minuta and acetic acid production in an anaerobic membrane bioreactor for sludge fermentation. Bioresour. Technol. 282, 197–201 (2019)
Lim, J.W., Chiam, J.A., Wang, J.Y.: Microbial community structure reveals how microaeration improves fermentation during anaerobic co-digestion of brown water and food waste. Bioresour. Technol. 171, 132–138 (2014)
Mariakakis, I., Bischoff, P., Krampe, J., Meyer, C., Steinmetz, H.: Effect of organic loading rate and solids retention time on microbial population during bio-hydrogen production by dark fermentation in large lab-scale. Int. J. Hydrog. Energy 36, 10690–10700 (2011)
Cohen, A., Distel, B., Van Deursen, A., Breure, A.M., Van Andel, J.G., Andel, V.: Role of anaerobic spore-forming bacteria in the acidogenesis of glucose: changes induced by discontinuous or low-rate feed supply. Antonie Van Leeuwenhoek 51, 179–192 (1985)
Castelló, E., García y Santos, C., Iglesias, T., Paolino, G., Wenzel, J., Borzacconi, L., Etchebehere, C.: Feasibility of biohydrogen production from cheese whey using a UASB reactor: links between microbial community and reactor performance. Int. J. Hydrog. Energy 34, 5674–5682 (2009)
Lan, G.Q., Ho, Y.W., Abdullah, N.: Mitsuokella jalaludinii sp. nov., from the rumens of cattle in Malaysia. Int. J. Syst. Evol. Microbiol. 52, 713–718 (2002)
Cheng, W., Chen, H., Yan, S.H., Su, J.: Illumina sequencing-based analyses of bacterial communities during short-chain fatty-acid production from food waste and sewage sludge fermentation at different pH values. World J. Microbiol. Biotechnol. 30, 2387–2395 (2014)
Weimer, P.J., Moen, G.N.: Quantitative analysis of growth and volatile fatty acid production by the anaerobic ruminal bacterium Megasphaera elsdenii T81. Appl. Microb. Cell Physiol. (2022). https://doi.org/10.1007/s00253-012-4645-4
Moreno-Andrade, I., Moreno, G., Kumar, G., Buitrón, G.: Biohydrogen production from industrial wastewaters. Water Sci. Technol. 71, 105–110 (2015)
Cabrol, L., Marone, A., Tapia-Venegas, E., Steyer, J.P., Ruiz-Filippi, G., Trably, E.: Microbial ecology of fermentative hydrogen producing bioprocesses: useful insights for driving the ecosystem function. FEMS Microbiol. Rev. 41, 158–181 (2017)
Feng, K., Li, H., Zheng, C.: Shifting product spectrum by pH adjustment during long-term continuous anaerobic fermentation of food waste. Bioresour. Technol. 270, 180–188 (2018)
Rivière, D., Desvignes, V., Pelletier, E., Chaussonnerie, S., Guermazi, S., Weissenbach, J., Li, T., Camacho, P., Sghir, A.: Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. ISME J. 3, 700–714 (2009)
Debroas, D., Blanchart, G.: Interactions between proteolytic and cellulolytic rumen bacteria during hydrolysis of plant cell wall protein. Reprod. Nutr. Dev. 33, 283–288 (1993)
Muszyński, A., Tabernacka, A., Miłobedzka, A.: Long-term dynamics of the microbial community in a full-scale wastewater treatment plant. Int. Biodeterior. Biodegrad. 100, 44–51 (2015)
Tang, J., Wang, X.C., Hu, Y., Pu, Y., Huang, J., Hao Ngo, H., Zeng, Y., Li, Y.: Nitrogen removal enhancement using lactic acid fermentation products from food waste as external carbon sources: performance and microbial communities. Bioresour. Technol. 256, 259–268 (2018)
Hu, H., Ma, S., Zhang, X., Ren, H.: Characteristics of dissolved organic nitrogen in effluent from a biological nitrogen removal process using sludge alkaline fermentation liquid as an external carbon source. Water Res. 176, 115741 (2020)
Ping, Q., Lu, X., Li, Y., Mannina, G.: Effect of complexing agents on phosphorus release from chemical-enhanced phosphorus removal sludge during anaerobic fermentation. Bioresour. Technol. 301, 122745 (2020)
Oren, A.: The Family Rhodocyclaceae. In: Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F. (eds.) The Prokaryotes: Alphaproteobacteria and Betaproteobacteria, 4th edn., pp. 976–994. Springer, Berlin (2014)
Mao, Y., Xia, Y., Zhang, T.: Characterization of Thauera-dominated hydrogen-oxidizing autotrophic denitrifying microbial communities by using high-throughput sequencing. Bioresour. Technol. 128, 703–710 (2013)
Foss, S., Harder, J.: Thauera linaloolentis sp. nov. and Thauera terpenica sp. nov., isolated on oxygen-containing monoterpenes (linalool, menthol, and eucalyptol and nitrate). Syst. Appl. Microbiol. 21, 365–373 (1998)
Liu, B., Zhang, F., Feng, X., Liu, Y., Yan, X., Zhang, X., Wang, L., Zhao, L.: Thauera and Azoarcus as functionally important genera in a denitrifying quinoline-removal bioreactor as revealed by microbial community structure comparison. FEMS Microbiol. Ecol. (2005). https://doi.org/10.1111/j.1574.6941.2005.00033.x
Nastro, R.A., Falcucci, G., Minutillo, M., Jannelli, E.: Microbial fuel cells in solid waste valorization: trends and applications. Model. Trends Solid Hazard. Waste Manag. 2017, 159–171 (2017)
Geesink, P., Taubert, M., Jehmlich, N., von Bergen, M., Küsel, K.: Bacterial necromass is rapidly metabolized by heterotrophic bacteria and supports multiple trophic levels of the groundwater microbiome. Microbiol. Spectr. (2022). https://doi.org/10.1128/spectrum.00437-22
Sydow, A., Krieg, T., Mayer, F., Schrader, J., Holtmann, D.: Electroactive bacteria—molecular mechanisms and genetic tools. Appl. Microbiol. Biotechnol. 98, 8481–8495 (2014)
Olsen, I., Johnson, J.L., Moore, L.V.H., Moore, W.E.C.: Lactobacillus uli sp. nov. and Lactobacillus rimae sp. nov. from the human gingival crevice and emended descriptions of Lactobacillus minutus and Streptococcus parvulus. Int. J. Syst. Bacteriol. 41, 261–266 (1991)
Li, S.L., Lin, J.S., Wang, Y.H., Lee, Z.K., Kuo, S.C., Tseng, I.C., Cheng, S.S.: Strategy of controlling the volumetric loading rate to promote hydrogen-production performance in a mesophilic-kitchen-waste fermentor and the microbial ecology analyses. Bioresour. Technol. 102, 8682–8687 (2011)
Weimer, P.J.: Redundancy, resilience, and host specificity of the ruminal microbiota: implications for engineering improved ruminal fermentations. Front. Microbiol. (2015). https://doi.org/10.3389/fmicb.2015.00296
Acknowledgements
The Green Propulsion Laboratory of Veritas S.p.A. is gratefully acknowledged for its hospitality.
Funding
This work was supported by the project “Ecopolimeri” (ID 10217222) in the frame of the POR-FESR 2014–2020 program of Regione Veneto.
Author information
Authors and Affiliations
Contributions
AL: Investigation, Data curation, Formal analysis, Writing—original draft. BC: Formal analysis, Writing—review and editing. GT: Resources, Funding acquisition. CC: Supervision, Conceptualization, Resources, Writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lanfranchi, A., Chouaia, B., Tassinato, G. et al. Microbial Community of the Acidogenic Fermentation of Urban Waste: Effect of the Hydrodynamic Cavitation Pre-treatment. Waste Biomass Valor 15, 1629–1639 (2024). https://doi.org/10.1007/s12649-023-02196-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02196-3