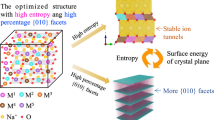
Abstract
Mn-based layered oxides are among the most promising cathode materials for sodium-ion batteries owing to the advantages of abundance, environmental friendliness, low cost and high specific capacity. P2 and O′3 are two representative structures of Mn-based layered oxides. However, the P2 structure containing insufficient Na generally exhibits low initial charge capacity, while O′3 structure with sufficient Na delivers high initial charge capacity but poor cycle stability. This study prepared a multitude of NaxMnO2 (x = 0.7, 0.8, 0.9) cathode materials with varying P2/O′3 ratios and further investigated their electrochemical performances. The optimized Na0.8MnO2, comprising 69.9 wt% O′3 and 30.1 wt% P2 phase, exhibited relatively balanced specific capacity, Coulombic efficiency and cycle stability. Specifically, it achieved a high specific capacity of 128.9 mAh·g−1 with an initial Coulombic efficiency of 98.2% in half-cell configuration. The Na0.8MnO2//hard carbon full cell also achieved a high specific capacity of 126.7 mAh·g−1 with an initial Coulombic efficiency of 98.9%. Moreover, the capacity fading mechanism was revealed by combining in-situ and ex-situ X-ray diffraction. The findings of this study provide theoretical guidance for further modification design of Mn-based layered cathodes.
Graphical abstract

摘要
锰基层状氧化物具有资源丰富、环保、低成本、高比容量等优点, 是钠离子电池中极具发展前景的正极材料之一。P2和O′3相是锰基层状氧化物中的两种代表性结构。然而, 含Na不足的P2相结构通常具有较低的初始充电容量, 而含有足量Na的O′3相结构虽具有较高的初始充电容量, 但其循环稳定性较差。本研究工作制备了多种不同P2/O′3比例的NaxMnO2(x = 0.7, 0.8, 0.9) 正极材料, 并进一步研究其电化学性能。优化的Na0.8MnO2正极材料 (其中O′3相含量为69.9 wt%, P2相含量为30.1 wt%) 具有相对平衡的比容量、库仑效率和循环稳定性。具体来说, 它在半电池配置下实现了128.9 mAh·g−1的高比容量和98.2%的初始库仑效率。Na0.8MnO2//硬碳全电池可实现126.7 mAh·g−1的高比容量, 初始库仑效率达到了98.9%。此外, 通过结合原位和非原位X射线衍射, 揭示了容量衰退机制。研究结果为锰基层状正极的进一步改性设计提供了理论指导。





Similar content being viewed by others
References
Lee J, Kitchaev DA, Kwon DH, Lee CW, Papp JK, Liu YS, Lun ZY, Clement RJ, Shi T, McCloskey BD, Guo JH, Balasubramanian M, Ceder G. Reversible Mn2+/Mn4+ double redox in lithium-excess cathode materials. Nature. 2018;556(7700):185. https://doi.org/10.1038/s41586-018-0015-4.
Xie J, Lu YC. A retrospective on lithium-ion batteries. Nat Commun. 2020;11(1):2499. https://doi.org/10.1038/s41467-020-16259-9.
Luo MH, Yu HX, Hu FY, Liu TT, Cheng X, Zheng RT, Bai Y, Shui M, Shu J. Metal selenides for high performance sodium ion batteries. Chem Eng J. 2020;380:122557. https://doi.org/10.1016/j.cej.2019.122557.
Li YM, Lu YX, Zhao CL, Hu YS, Titirici MM, Li H, Huang XJ, Chen LQ. Recent advances of electrode materials for low-cost sodium-ion batteries towards practical application for grid energy storage. Energy Storage Mater. 2017;7:130. https://doi.org/10.1016/j.ensm.2017.01.002.
Kim J, Kim J, Jeong JY, Park J, Park CY, Park S, Lim SG, Lee KT, Choi NS, Byon HR, Jo CS, Lee J. Designing fluorine-free electrolytes for stable sodium metal anodes and high-power seawater batteries via SEI reconstruction. Energy Environ Sci. 2022;15(10):4109. https://doi.org/10.1039/D2EE01295B.
Wang YS, Wang QC, Ding XY, Wang M, Xin YH, Gao HC. The nitrogen-doped carbon coated Na4MnV(PO4)3 as a high electrochemical performance cathode material for sodium-ion batteries. Appl Surf Sci. 2022;601:154218. https://doi.org/10.1016/j.apsusc.2022.154218.
Huang CC, Liu YW, Zheng RT, Yang ZW, Miao ZH, Zhang JW, Cai XH, Yu HX, Zhang LY, Shu J. Interlayer gap widened TiS2 for highly efficient sodium-ion storage. J Mater Sci Tech. 2022;107:64. https://doi.org/10.1016/j.jmst.2021.08.035.
Pan HL, Hu YS, Chen LQ. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ Sci. 2013;6(8):2338. https://doi.org/10.1039/c3ee40847g.
Bin D, Wang F, Tamirat AG, Suo LM, Wang YG, Wang CS, Xia YY. Progress in aqueous rechargeable sodium-ion batteries. Adv Energy Mater. 2018;8(17):1703008. https://doi.org/10.1002/aenm.201703008.
Xie BY, Zuo PJ, Wang LG, Wang JJ, Huo H, He MX, Shu J, Li HF, Lou SF, Yin GP. Achieving long-life Prussian blue analogue cathode for Na-ion batteries via triple-cation lattice substitution and coordinated water capture. Nano Energy. 2019;61:201. https://doi.org/10.1016/j.nanoen.2019.04.059.
Zuo WH, Innocenti A, Zarrabeitia M, Bresser D, Yang Y, Passerini S. Layered oxide cathodes for sodium-ion batteries: storage mechanism, electrochemistry, and techno-economics. Acc Chem Res. 2023;56(3):284. https://doi.org/10.1021/acs.accounts.2c00690.
Baumann M, Häringer M, Schmidt M, Schneider L, Peters JF, Bauer W, Binder JR, Weil M. Prospective sustainability screening of sodium-ion battery cathode materials. Adv Energy Mater. 2022;12(46):2202636. https://doi.org/10.1002/aenm.202202636.
Chen H, Xu BB, Ping QS, Wu BZ, Wu XK, Zhuang QQ, Wang HL, Wang BF. Co2B2O5 as an anode material with high capacity for sodium ion batteries. Rare Met. 2020;39(9):1045. https://doi.org/10.1007/s12598-020-01383-8.
Du M, Du KD, Guo JZ, Liu Y, Aravindan V, Yang JL, Zhang KY, Gu ZY, Wang XT, Wu XL. Direct reuse of oxide scrap from retired lithium-ion batteries: advanced cathode materials for sodium-ion batteries. Rare Met. 2023;42(5):1603. https://doi.org/10.1007/s12598-022-02230-8.
Ou C, Tan MD, Li ZB, Li ZJ, Qiao TF, ZP, Lei DN, Wang CX,. Carbon-coated hybrid crystals with fast electrochemical reaction kinetics for ultra-stable and high-load sodium-ion batteries. Rare Met. 2023. https://doi.org/10.1016/j.cej.2023.143032.
Liang K, Zhao HS, Li JB, Huang XB, Jia SY, Chen WK, Ren YR. Engineering crystal growth and surface modification of Na3V2(PO4)2F3 cathode for high-energy-density sodium-ion batteries. Small. 2023;19(19):e2207562. https://doi.org/10.1002/smll.202207562.
Zhao HS, Qi YL, Liang K, Zhu WK, Wu HB, Li JB, Ren YR. Phosphorus-doping and oxygen vacancy endowing anatase TiO2 with excellent sodium storage performance. Rare Met. 2021;41(4):1284. https://doi.org/10.1007/s12598-021-01864-4.
Nanba Y, Iwao T, Boisse BMD, Zhao WW, Hosono E, Asakura D, Niwa H, Kiuchi H, Miyawaki J, Harada Y, Okubo M, Yamada A. Redox potential paradox in NaxMO2 for sodium-ion battery cathodes. Chem Mater. 2016;28(4):1058. https://doi.org/10.1021/acs.chemmater.5b04289.
You Y, Manthiram A. Progress in high-voltage cathode materials for rechargeable sodium-ion batteries. Adv Energy Mater. 2018;8(2):1701785. https://doi.org/10.1002/aenm.201701785.
Zhang ZW, Zhong XB, Zhang YH, Tang MY, Li SX, Zhang HH, Hu PF, Liang JF. Scalable synthesis of mesoporous FeS2 nanorods as high-performance anode materials for sodium-ion batteries. Rare Met. 2022;41(1):21. https://doi.org/10.1007/s12598-021-01835-9.
Su H, Jaffer S, Yu HJ. Transition metal oxides for sodium-ion batteries. Energy Storage Mater. 2016;5:116. https://doi.org/10.1016/j.ensm.2016.06.005.
Fang C, Huang YH, Zhang WX, Han JT, Deng Z, Cao YL, Yang HX. Routes to high energy cathodes of sodium-ion batteries. Adv Energy Mater. 2016;6(5):1501727. https://doi.org/10.1002/aenm.201501727.
Deng JQ, Luo WB, Chou SL, Liu HK, Dou SX. Sodium-ion batteries: from academic research to practical commercialization. Adv Energy Mater. 2018;8(4):1701428. https://doi.org/10.1002/aenm.201701428.
Peng B, Wan GL, Ahmad N, Yu L, Ma XY, Zhang GQ. Recent progress in the emerging modification strategies for layered oxide cathodes toward practicable sodium ion batteries. Adv Energy Mater. 2023;13(27):2300334. https://doi.org/10.1002/aenm.202300334.
Peng B, Chen YX, Wang F, Sun ZH, Zhao LP, Zhang XL, Wang WT, Zhang GQ. Unusual Site-selective doping in layered cathode strengthens electrostatic cohesion of alkali-metal layer for practicable sodium-ion full cell. Adv Mater. 2022;34(6):2103210. https://doi.org/10.1002/adma.202103210.
Sun YY, Li SQ, Wang CR, Qian YX, Zheng SY, Tao Y. Research progress of layered transition metal oxide cathode materials for sodium ion batteries. Chin J Rare Met. 2023;46(6):776. https://doi.org/10.13373/j.cnki.cjrm.XY22020014.
Tian JL, Wu LR, Zhao HJ, Xu SD, Chen L, Zhang D, Duan XC. Development of lithium-free P2-type high-sodium content cathode materials with enhanced cycle and air stability for sodium-ion batteries. Rare Met. 2023. https://doi.org/10.1007/s12598-023-02422-w.
Wang F, Peng B, Zeng SY, Zhao LP, Zhang XL, Wan GL, Zhang HL, Zhang GQ. Activating oxygen redox in layered NaXMnO2 to suppress intrinsic deficient behavior and enable phase-transition-free sodium ion cathode. Adv Funct Mater. 2022;32(35):2202665. https://doi.org/10.1002/adfm.202202665.
Liu GQ, Li Y, Du YL, Wen L. Synthesis and properties of Na0.8Ni0.4Mn0.6O2 oxide used as cathode material for sodium ion batteries. Rare Met. 2017;36(12):977. https://doi.org/10.1007/s12598-016-0757-9.
Su GQ, Li LJ, Shi Z, Ma XB, Ma L, Cao ZJ. Boosting anionic redox through lithium doping in P2-layered cathode for high-performance sodium-ion batteries. Appl Surf Sci. 2023;608:155097. https://doi.org/10.1016/j.apsusc.2022.155097.
Gu FP, Yao XL, Sun TJ, Fang MH, Shui M, Shu J, Ren Y. Studies on micron-sized Na0.7MnO2.05 with excellent cycling performance as a cathode material for aqueous rechargeable sodium-ion batteries. Appl Phys A. 2020;126(8):658. https://doi.org/10.1007/s00339-020-03799-6.
Zhou JK, Liu J, Li YY, Zhao ZJ, Zhou PF, Wu XZ, Tang XN, Zhou J. Reaching the initial coulombic efficiency and structural stability limit of P2/O3 biphasic layered cathode for sodium-ion batteries. J Colloid Interf Sci. 2023;638:758. https://doi.org/10.1016/j.jcis.2023.02.001.
Delmas C, Fouassier C, Hagenmuller P. Structural classification and properties of the layered oxides. Phys B+c. 1980;99(1–4):81. https://doi.org/10.1016/0378-4363(80)90214-4.
Wang X, Dong XP, Feng XC, Shi QH, Wang J, Yin XM, Zhang JJ, Zhao YF. In-Plane BO3 configuration in P2 layered oxide enables outstanding long cycle performance for sodium ion batteries. Small Methods. 2023;7(1):e2201201. https://doi.org/10.1002/smtd.202201201.
Ortiz-Vitoriano N, Drewett NE, Gonzalo E, Rojo T. High performance manganese-based layered oxide cathodes: overcoming the challenges of sodium ion batteries. Energy Environ Sci. 2017;10(5):1051. https://doi.org/10.1039/C7EE00566K.
Cao YG, Xiao MJ, Sun XZ, Dong WJ, Huang FQ. Recent advances on high-capacity sodium manganese-based oxide cathodes for sodium-ion batteries. Chem Eur J. 2023;29(12):e202202997. https://doi.org/10.1002/chem.202202997.
Bucher N, Hartung S, Nagasubramanian A, Cheah YL, Hoster HE, Madhavi S. Layered NaxMnO2+z in sodium ion batteries-influence of morphology on cycle performance. ACS Appl Mater Interfaces. 2014;6(11):8059. https://doi.org/10.1021/am406009t.
Liu XS, Zuo WH, Zheng BZ, Xiang YX, Zhou K, Xiao ZM, Shan PZ, Shi JW, Li Q, Zhong GM, Fu RQ, Yang Y. P2-Na0.67AlxMn1−xO2: cost-effective, stable and high-rate sodium electrodes by suppressing phase transitions and enhancing sodium cation mobility. Angew Chem Int Ed. 2019;58(50):18086. https://doi.org/10.1002/anie.201911698.
Ma XH, Chen HL, Ceder G. Electrochemical properties of monoclinic NaMnO2. J Electrochem Soc. 2011;158(12):A1307. https://doi.org/10.1149/2.035112jes.
Mendiboure A, Delmas C, Hagenmuller P. Electrochemical intercalation and deintercalation of NaxMnO2 bronzes. J Solid State Chem. 1985;57(3):323. https://doi.org/10.1016/0022-4596(85)90194-X.
Wang YX, Wang LG, Zhu H, Chu J, Fang YJ, Wu LN, Huang L, Ren Y, Sun CJ, Liu Q, Ai XP, Yang HX, Cao YL. Ultralow-strain Zn-substituted layered oxide cathode with suppressed P2–O2 transition for stable sodium ion storage. Adv Funct Mater. 2020;30(13):1910327. https://doi.org/10.1002/adfm.201910327.
Sato T, Sato K, Zhao WW, Kajiya Y, Yabuuchi N. Metastable and nanosize cation-disordered rocksalt-type oxides: revisit of stoichiometric LiMnO2 and NaMnO2. J Mater Chem A. 2018;6(28):13943. https://doi.org/10.1039/c8ta03667e.
Li JY, Hu HY, Zhou LF, Li HW, Lei YJ, Lai WH, Fan YM, Zhu YF, Peleckis G, Chen SQ, Pang WK, Peng J, Wang JZ, Dou SX, Chou SL, Xiao Y. Surface lattice-matched engineering based on in situ spinel interfacial reconstruction for stable heterostructured sodium layered oxide cathodes. Adv Funct Mater. 2023;33(14):2213215. https://doi.org/10.1002/adfm.202213215.
Li JY, Lü HY, Zhang XH, Xing YM, Wang G, Guan HY, Wu XL. P2-type Na0.53MnO2 nanorods with superior rate capabilities as advanced cathode material for sodium ion batteries. Chem Eng J. 2017;316:499. https://doi.org/10.1016/j.cej.2017.01.109.
Palluzzi M, Silvestri L, Celeste A, Tuccillo M, Latini A, Brutti S. Structural degradation of O3-NaMnO2 positive electrodes in sodium-ion batteries. Crystals. 2022;12(7):885. https://doi.org/10.3390/cryst12070885.
Zhang GH, Li JY, Fan YX, Liu YK, Zhang P, Shi XY, Ma JW, Zhang RY, Huang YH. Suppressed P2–P2′ phase transition of Fe/Mn-based layered oxide cathode for high-performance sodium-ion batteries. Energy Storage Mater. 2022;51:559. https://doi.org/10.1016/j.ensm.2022.06.045.
Zhang Y, Wu MM, Ma JW, Wei GF, Ling Y, Zhang RY, Huang YH. Revisiting the Na2/3Ni1/3Mn2/3O2 cathode: oxygen redox chemistry and oxygen release suppression. ACS Cent Sci. 2020;6(2):232. https://doi.org/10.1021/acscentsci.9b01166.
Wei P, Sun XP, He ZM, Cheng FY, Xu J, Li Q, Ren YR, He JH, Han JT, Huang YH. Adenosine triphosphate induced transition-metal phosphide nanostructures encapsulated with N, P-codoped carbon toward electrochemical water splitting. Fuel. 2023;339:127303. https://doi.org/10.1016/j.fuel.2022.127303.
Peng B, Sun ZH, Jiao SH, Li J, Wang GR, Li YP, Jin X, Wang XQ, Li JM, Zhang GQ. Facile self-templated synthesis of P2-type Na0.7CoO2 microsheets as a long-term cathode for high-energy sodium-ion batteries. J Mater Chem A. 2019;7(23):13922. https://doi.org/10.1039/c9ta02966d.
Zhang W, Sun XL, Tang YX, Xia HR, Zeng Y, Qiao L, Zhu ZQ, Lv ZS, Zhang YY, Ge X, Xi SB, Wang ZG, Du YH, Chen XD. Lowering charge transfer barrier of LiMn2O4 via nickel surface doping to enhance li+ intercalation kinetics at subzero temperatures. J Am Chem Soc. 2019;141(36):14038. https://doi.org/10.1021/jacs.9b05531.
Hwang JY, Kim J, Yu TY, Sun YK. A new P2-type layered oxide cathode with extremely high energy density for sodium-ion batteries. Adv Energy Mater. 2019;9(15):1803346. https://doi.org/10.1002/aenm.201803346.
Li LJ, Su GQ, Lu C, Ma XB, Ma L, Wang HL, Cao ZJ. Effect of lithium doping in P2-type layered oxide cathodes on the electrochemical performances of Sodium-Ion batteries. Chem Eng J. 2022;446:136923. https://doi.org/10.1016/j.cej.2022.136923.
Zhao CL, Yao ZP, Wang QD, Li HF, Wang JL, Liu M, Ganapathy S, Lu YX, Cabana J, Li BH, Bai XD, Aspuru-Guzik A, Wagemaker M, Chen LQ, Hu YS. Revealing high Na-content P2-type layered oxides as advanced sodium-ion cathodes. J Am Chem Soc. 2020;142(12):5742. https://doi.org/10.1021/jacs.9b13572.
Kubota K, Miyazaki M, Kim EJ, Yoshida H, Barpanda P, Komaba S. Structural change induced by electrochemical sodium extraction from layered O’3-NaMnO2. J Mater Chem A. 2021;9(47):26810. https://doi.org/10.1039/D1TA05390F.
Acknowledgements
This study was financially supported by the Natural Science Research Project of Anhui Province Education Department (No. 2022AH050334), the Scientific Research Foundation of Anhui University of Technology for Talent Introduction (No. DT2200001211) and the New Energy Electric Vehicles High-Voltage Components Inspection and Testing Public Service Platform.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, B., Zhou, ZH., Xuan, P. et al. Realizing high initial Coulombic efficiency in manganese-based layered oxide cathodes for sodium-ion batteries via P2/O′3 biphasic structure optimization. Rare Met. 43, 2093–2102 (2024). https://doi.org/10.1007/s12598-023-02581-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02581-w