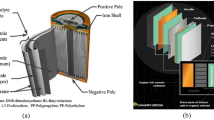
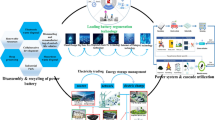
Abstract
The lithium-ion batteries (LIBs) have been widely equipped in electric/hybrid electric vehicles (EVs/HEVs) and the portable electronics due to their excellent electrochemical performances. However, a large number of retired LIBs that consist of toxic substances (e.g., heavy metals, electrolytes) and valuable metals (e.g., Li, Co) will inevitably flow into the waste stream, and their incineration or landfill treatment will cause severe risks to ecosystem and human beings. The sustainable and efficient treatment or recycling of valuable resources from spent LIBs should be fully recognized for environmental and resource security. As one of the most important processes for spent LIBs recycling, the pretreatment is an indispensable step, which is directly related to the subsequent metal extraction and separation processes. Although considerable progresses have been made regarding the pretreatment technologies, there are few summarized reports concerning critical processes of spent LIBs recycling, especially combination of currently available recycling technologies with industrialized applications during pretreatments. Therefore, comprehensive review of the current prevailing pretreatment technologies in laboratory to existing scale-up applications is quite necessary to reveal cutting-edge development in the field of pretreatment. In this review, the current pretreatment technologies are systematically categorized and introduced, along with critical discussions. This review focused on the various options for pretreatment processes itself, instead of general spent LIBs recycling technologies without the focused topics that have been sophisticatedly reviewed by previous studies. Here, the deactivation, discharge, dismantling, separation, liberation of active material and electrolyte treatment have been summarized with the in-depth discussion of the technology development and current status of each category. Finally, current states of industrial development are also reviewed and discussed for the development of efficient and environmentally friendly recycling technologies for future applications. This review tends to present a focused topic concerning the pretreatment of spent LIBs to potential readers with a comprehensive illustration of the development on both cutting-edge technologies and scale-up applications.
Graphical abstract

摘要
锂离子电池(LIBs)由于其出色的电化学性能,已经被广泛地装备在电动/混合电动汽车(EVs/HEVs)和便携式电子产品中。然而,退役LIBs中含有大量的有毒物质(如重金属、电解质)和有价金属(如锂、钴),焚烧或填埋处理将对生态系统和人类造成严重风险。为了环境和资源的安全,可持续和有效地处理或回收废旧LIBs中的宝贵资源是十分重要的。预处理作为废LIBs回收的最重要、不可或缺的过程之一,其好坏直接关系到后续的金属提取和分离过程。尽管在预处理技术方面已经取得了相当大的进展,但有关废旧LIBs回收预处理过程的关键过程的总结却很少,特别是在预处理过程中把目前可用的回收技术与工业化应用相结合。因此,本文全面回顾目前在实验室中流行的预处理技术和现有的放大应用,以揭示预处理领域的前沿发展。在本综述中,对当前的预处理技术进行了系统的分类和介绍,并进行了重要的讨论。本综述侧重于预处理过程的各种方法,而不是介绍一般的废LIBs的回收技术,这在之前的综述中很少见。在这里,总结了失活、放电、拆解、分离、活性材料与集流体的分离和电解质处理等处理技术,并对每一类的技术发展和现状进行了深入讨论。最后,还回顾和讨论了工业发展的现状,以便为未来的应用开发出高效和环保的回收技术。本文倾向于向潜在的读者介绍有关废旧LIBs预处理部分,全面说明前沿技术和放大应用的发展。





Reproduced with permission from Ref. [11]. Copyright 2022, Elsevier

Reproduced with permission from Ref. [71]. Copyright 2014, Elsevier. b Particles and aggregates existing in Magnetic-Waterflow separation. Reproduced with permission from Ref. [88]. Copyright 2021, Elsevier. c Sample of granule mixture and corona electrostatic separator (CES) in electrostatic separation. Reproduced with permission from Ref. [91]. Copyright 2020, Sage. d Schematic diagram for graphite and LCO separation using Fenton reagent-assisted flotation. Reproduced with permission from Ref. [100]. Copyright 2017, Elsevier. e Separation diagram of eddy current. Reproduced with permission from Ref. [104]. Copyright 2019, Elsevier. f Schematic of pneumatic separation of spent LIBs in industrial process. Reproduced with permission from Ref. [106]. Copyright 2020, Elsevier

Reproduced with permission from Ref. [118]. Copyright 2015, Elsevier. b Flowchart for cathode separation by using CaO as heat treatment medium and possible reaction mechanism for PVDF. Reproduced with permission from Ref. [66]. Copyright 2019, ACS. c Separation of active materials from current collectors after pyrolysis. Reproduced with permission from Ref. [123]. Copyright 2016, Elsevier. d Digital pictures of cathode after different treatments under a CO2 atmosphere at 600 °C. Reproduced with permission from Ref. [115]. Copyright 2022, RSC. e Pyrolysis mechanism of cathode at different temperatures. Reproduced with permission from Ref. [125]. Copyright 2022, Elsevier. f Schematic illustration of separating cathode materials and Al foils by low-temperature molten salt (AlCl3-NaCl). Reproduced with permission from Ref. [128]. Copyright 2019, ACS

Reproduced with permission from Ref. [137]. Copyright 2015, RSC. b Diagram of peeling process and peeling off efficiency by heating ILs ([BMIm][BF4]). Reproduced with permission from Ref. [144]. Copyright 2014, Elsevier. c Schematic of recycling and diagram of DES (choline chloride-ethylene glycol). Reproduced with permission from Ref. [21]. Copyright 2019, Nature. d Schematic of methyl ester solvent synthesis and cathode materials/Al foils separation. Reproduced with permission from Ref. [136]. Copyright 2020, ACS. e Scheme and schematic representation of PVDF dissolution in SC CO2 process. Reproduced with permission from Ref. [154]. Copyright 2021, Elsevier. f Peeling off mechanism with ultrasound-assisted Fenton reaction system. Reproduced with permission from Ref. [135]. Copyright 2021, Elsevier

Reproduced with permission from Ref. [171]. Copyright 2019, Elsevier. b Schematic illustration of reclaimed electrolytes from spent LIBs. Reproduced with permission from Ref. [178]. Copyright 2017, ACS. c Schematic of low-temperature volatilization. Reproduced with permission from Ref. [23]. Copyright 2020, Elsevier. d Schematic illustration of lab-scale pyrolysis system. Reproduced with permission from Ref. [173]. Copyright 2011, Elsevier
Similar content being viewed by others
References
Fan ES, Li L, Wang ZP, Lin J, Huang YX, Yao Y, Chen RJ, Wu F. Sustainable recycling technology for Li-ion batteries and beyond: challenges and future prospects. Chem Rev. 2020;120(14):7020. https://doi.org/10.1021/acs.chemrev.9b00535.
Natarajan S, Aravindan V. Burgeoning prospects of spent lithium-ion batteries in multifarious applications. Adv Energy Mater. 2018;8(33):1802303. https://doi.org/10.1002/aenm.201802303.
Ma LX, Chen TD, Hai CX, Dong SD, He X, Xu Q, Feng H, Xin A, Chen JT, Zhou Y. Surface engineering of Li- and Mn-rich layered oxides for superior Li-ion battery. Tungsten. 2022. https://doi.org/10.1007/s42864-022-00187-w.
Zhao Y, Fang LZ, Kang YQ, Wang L, Zhou YN, Liu XY, Li T, Li YX, Liang Z, Zhang ZX, Li BH. A novel three-step approach to separate cathode components for lithium-ion battery recycling. Rare Met. 2021;40(6):1431. https://doi.org/10.1007/s12598-020-01587-y.
Zhang ZQ, Li L, Li X, Hu YC, Huang K, Xue BY, Wang YQ, Yu YJ. State-of-health estimation for the lithium-ion battery based on gradient boosting decision tree with autonomous selection of excellent features. Int J Energy Res. 2022;46(2):1756. https://doi.org/10.1002/er.7292.
Lv WG, Wang ZH, Cao HB, Sun Y, Zhang Y, Sun Z. A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain Chem Eng. 2018;6(2):1504. https://doi.org/10.1021/acssuschemeng.7b03811.
Fan MC, Wozny J, Gong J, Kang YQ, Wang XS, Zhang ZX, Zhou GM, Zhao Y, Li BH, Kang FY. Lithium metal recycling from spent lithium-ion batteries by cathode overcharging process. Rare Met. 2022;41(6):1843. https://doi.org/10.1007/s12598-021-01918-7.
Li WB, Wu K, Feng H, Wang N, Zhang JH, Wang JJ, Li XF. Atomic layer deposition of ultrafine Pd nanoparticles for enhancing the rate capability of LiNi0.8Co0.1Mn0.1O2 cathode. Tungsten. 2022;4(4):346. https://doi.org/10.1007/s42864-022-00178-x.
Yu DW, Huang Z, Makuza B, Guo XY, Tian QH. Pretreatment options for the recycling of spent lithium-ion batteries: a comprehensive review. Miner Eng. 2021;173:107218. https://doi.org/10.1016/j.mineng.2021.107218.
Assefi M, Maroufi S, Yamauchi Y, Sahajwalla V. Pyrometallurgical recycling of Li-ion, Ni-Cd and Ni-MH batteries: a mini-review, current opinion in green and sustainable. Chemistry. 2020;24:26. https://doi.org/10.1016/j.cogsc.2020.01.005.
SNE research, 2022. https://www.sneresearch.com/kr/home/ (Accessed Aug 1, 2022).
Ggii. Power battery shipments of China, 2022. https://www.gg-lb.com/art-42171.html (Accessed Aug 1, 2022).
Zhang JR, Lan ZW, Xi RH, Li YY, Wang JT, Zhang Caihong. Review on deficiency and modification of high nickel ternary materials for lithium-ion batteries. Chin J of Rare Met. 2022,46(3):367. https://doi.org/10.13373/j.cnki.cjrm.XY20090004.
Li M, Cheng LL, Yang YM, Niu F, Zhang XL, Liu DH. Development of technology for spent lithium-ion batteries recycling: a review. Chin J of Rare Met. 2022;46(3):349. https://doi.org/10.13373/j.cnki.cjrm.XY20020020.
Su MM, Huang G, Wang SQ, Wang YJ, Wang HH. High safety separators for rechargeable lithium batteries. Sci China-Chem. 2021;64(7):1131. https://doi.org/10.1007/s11426-021-1011-9.
Reddy MV, Mauger A, Julien CM, Paolella A, Zaghib K. Brief history of early lithium-battery development. Materials. 2020;13(8):1884. https://doi.org/10.3390/ma13081884.
Li JT, Wu ZY, Lu YQ, Zhou Y, Huang QS, Huang L, Sun SG. Water soluble binder, an electrochemical performance booster for electrode materials with high energy density. Adv Energy Mater. 2017;7(24):1701185. https://doi.org/10.1002/aenm.201701185.
He YQ, Yuan X, Zhang GW, Wang HF, Zhang T, Xie WN, Li LP. A critical review of current technologies for the liberation of electrode materials from foils in the recycling process of spent lithium-ion batteries. Sci Total Environ. 2020;766:142382. https://doi.org/10.1016/j.scitotenv.2020.142382.
Zhu T. Mechanics of high-capacity electrodes in lithium-ion batteries. Chin Phys B. 2016;25(1):014601. https://doi.org/10.1088/1674-1056/25/1/014601.
Liu ZF, Jiang YJ, Hu QM, Guo ST, Yu L, Li Q, Liu Q, Hu XL. Safer lithium-ion batteries from the separator aspect: development and future perspectives. Energy Environ Mater. 2021;4(3):336. https://doi.org/10.1002/eem2.12129.
Tran MK, Rodrigues MTF, Kato K, Babu G, Ajayan PM. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat Energy. 2019;4(4):339. https://doi.org/10.1038/s41560-019-0368-4.
Roy JJ, Rarotra S, Krikstolaityte V, Zhuoran KW, Cindy YDI, Tan XY, Carboni M, Meyer D, Yan Q, Srinivasan M. Green recycling methods to treat lithium-ion batteries E-waste: a circular approach to sustainability. Adv Mater. 2021. https://doi.org/10.1002/adma.202103346.
Zhong XH, Liu W, Han JW, Jiao F, Qin WQ, Liu T. Pretreatment for the recovery of spent lithium ion batteries: theoretical and practical aspects. J Clean Product. 2020;263:121439. https://doi.org/10.1016/j.jclepro.2020.121439.
Chagnes A, Pospiech B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J Chem Technol Biotechnol. 2013;88(7):1191. https://doi.org/10.1002/jctb.4053.
London metal exchange home page, 2022. https://www.lme.com/ (Accessed Aug 1, 2022).
Shanghai metals market new energy division home page. 2022. https://price.metal.com/ (Accessed Aug 1, 2022).
Zhang GW, Yuan X, He YQ, Wang HF, Zhang T, Xie WN. Recent advances in pretreating technology for recycling valuable metals from spent lithium-ion batteries. J Hazard Mater. 2021;406:124332. https://doi.org/10.1016/j.jhazmat.2020.124332.
Zhang XX, Li L, Fan ES, Xue Q, Bian YF, Wu F, Chen RJ. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem Soc Rev. 2018;47(19):7239. https://doi.org/10.1039/C8CS00297E.
Ordonez J, Gago EJ, Girard A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew Sustain Energy Rev. 2016;60:195. https://doi.org/10.1016/j.rser.2015.12.363.
Chaudhary V, Lakhera P, Kim KH, Deep A, Kumar P. Insights into the eco-friendly recovery process for valuable metals from waste lithium-ion batteries: organic acids leaching. Sep Purif Rev. 2022. https://doi.org/10.1080/15422119.2022.2164650.
Jiang YZ, Chen XP, Yan SX, Ou YD, Zhou T. Mechanochemistry-induced recycling of spent lithium-ion batteries for synergistic treatment of mixed cathode powders. Green Chem. 2022;24(15):5987. https://doi.org/10.1039/d2gc01929a.
Mousa E, Hu XF, Ye GZ. Effect of graphite on the recovery of valuable metals from spent Li-ion batteries in baths of hot metal and steel. Recycling. 2022;7(1):5. https://doi.org/10.3390/recycling7010005.
Murakami Y, Matsuzaki Y, Murakami K, Hiratani S, Shibayama A, Inoue R. Recovery rates of used rechargeable lithium-ion battery constituent elements in heat treatment. Metall Mater Trans B-Process Metall Mater Process Sci. 2020;51(4):1355. https://doi.org/10.1007/s11663-020-01834-8.
Pinna EG, Toro N, Gallegos S, Rodriguez MH. A novel recycling route for spent Li-ion batteries. Materials. 2022;15(1):44. https://doi.org/10.3390/ma15010044.
Zhu BW, Zhang YJ, Zou YL, Yang ZL, Zhang B, Zhao Y, Zhang MY, Meng Q, Dong P. Leaching kinetics and interface reaction of LiNi0.6Co0.2Mn0.2O2 materials from spent LIBs using GKB as reductant. J Environ Manag. 2021;300:113710. https://doi.org/10.1016/j.jenvman.2021.113710.
Zeng GS, Yao JX, Liu CL, Luo XB, Ji HY, Mi X, Deng CJ. Simultaneous recycling of critical metals and aluminum foil from waste LiNi1/3Co1/3Mn1/3O2 cathode via ethylene glycol-citric acid system. Acs Sustain Chem Eng. 2021;9(48):16133. https://doi.org/10.1021/acssuschemeng.1c04806.
Natarajan S, Boricha AB, Bajaj HC. Recovery of value-added products from cathode and anode material of spent lithium-ion batteries. Waste Manag. 2018;77:455. https://doi.org/10.1016/j.wasman.2018.04.032.
Jiang YZ, Chen XP, Yan SX, Li SZ, Zhou T. Pursuing green and efficient process towards recycling of different metals from spent lithium-ion batteries through ferro-chemistry. Chem Eng J. 2021;426:131637. https://doi.org/10.1016/j.cej.2021.131637.
Meng K, Cao Y, Zhang B, Ou X, Li DM, Zhang JF, Ji XB. Comparison of the ammoniacal leaching behavior of layered LiNixCoyMn1–x–yO2 (x = 1/3, 0.5, 0.8) cathode materials. ACS Sustain Chem Eng. 2019;7(8):7750. https://doi.org/10.1021/acssuschemeng.8b06675.
Qi YP, Meng FS, Yi XX, Shu JC, Chen MJ, Sun Z, Sun SH, Xiu FR. A novel and efficient ammonia leaching method for recycling waste lithium ion batteries. J Clean Product. 2020;251:119665. https://doi.org/10.1016/j.jclepro.2019.119665.
Roy JJ, Madhavi S, Cao B. Metal extraction from spent lithium-ion batteries (LIBs) at high pulp density by environmentally friendly bioleaching process. J Clean Product. 2021;280:124242. https://doi.org/10.1016/j.jclepro.2020.124242.
Ma YY, Zhou XY, Tang JJ, Liu XJ, Gan HX, Yang J. Reaction mechanism of antibiotic bacteria residues as a green reductant for highly efficient recycling of spent lithium-ion batteries. J Hazard Mater. 2021;417:126032. https://doi.org/10.1016/j.jhazmat.2021.126032.
Kang SK, Park JW, Tikue ET, Zhang HX, Yang S, Lee PS. Self-cross-linking nanocomposite membranes for green recycling of the solvent during lithium-ion battery manufacturing. Acs Sustain Chem Eng. 2022. https://doi.org/10.1021/acssuschemeng.1c06715.
He LP, Sun SY, Song XF, Yu JG. Recovery of cathode materials and Al from spent lithium-ion batteries by ultrasonic cleaning. Waste Manag. 2015;46:523. https://doi.org/10.1016/j.wasman.2015.08.035.
Li JH, Shi PX, Wang ZF, Chen Y, Chang CC. A combined recovery process of metals in spent lithium-ion batteries. Chemosphere. 2009;77(8):1132. https://doi.org/10.1016/j.chemosphere.2009.08.040.
Chan KH, Malik M, Azimi G. Separation of lithium, nickel, manganese, and cobalt from waste lithium-ion batteries using electrodialysis. Resour Conserv Recycl. 2022;178:106076. https://doi.org/10.1016/j.resconrec.2021.106076.
Widijatmoko SD, Gu F, Wang Z, Hall P. Selective liberation in dry milled spent lithium-ion batteries. Sustain Mater Technol. 2020;23:00134. https://doi.org/10.1016/j.susmat.2019.e00134.
Ma XT, Chen MY, Chen B, Meng ZF, Wang Y. High-performance graphite recovered from spent lithium-ion batteries. Acs Sustain Chem Eng. 2019;7(24):19732. https://doi.org/10.1021/acssuschemeng.9b05003.
Qin ZY, Wen ZX, Xu YF, Zheng ZC, Bai ML, Zhang N, Jia CK, Wu HB, Chen G. A ternary molten salt approach for direct regeneration of LiNi0.5Co0.2Mn0.3O2 cathode. Small. 2022;18(43):2106719. https://doi.org/10.1002/smll.202106719.
Xiao JF, Li J, Xu ZM. Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy. J Hazard Mater. 2017;338(15):124. https://doi.org/10.1016/j.jhazmat.2017.05.024.
Choi JW, Cho CW, Yun YS. Organic acid-based linear free energy relationship models for green leaching of strategic metals from spent lithium-ion batteries and improvement of leaching performance. J Hazard Mater. 2022;423:127214. https://doi.org/10.1016/j.jhazmat.2021.127214.
Peng C, Liu FP, Aji AT, Wilson BP, Lundstrom M. Extraction of Li and Co from industrially produced Li-ion battery waste - using the reductive power of waste itself. Waste Manage. 2019;95:604. https://doi.org/10.1016/j.wasman.2019.06.048.
Wang HY, Li ZF, Meng Q, Duan JG, Xu ML, Lin Y, Zhang YJ. Ammonia leaching of valuable metals from spent lithium ion batteries in NH3-(NH4)2SO4-Na2SO3 system. Hydrometallurgy. 2022;208:105809. https://doi.org/10.1016/j.hydromet.2021.105809.
Zheng HS, Dong T, Sha YF, Jiang DF, Zhang HT, Zhang SJ. Selective extraction of lithium from spent lithium batteries by functional ionic liquid. ACS Sustain Chem Eng. 2021;9(20):7022. https://doi.org/10.1021/acssuschemeng.1c00718.
Zhu P, Chen Y, Wang LY, Zhou M. Treatment of waste printed circuit board by green solvent using ionic liquid. Waste Manag. 2012;32(10):1914. https://doi.org/10.1016/j.wasman.2012.05.025.
Wang BY, Liu F, Zhang F, Tan M, Jiang HQ, Liu Y, Zhang Y. Efficient separation and recovery of cobalt(II) and lithium(I) from spent lithium ion batteries (LIBs) by polymer inclusion membrane electrodialysis (PIMED). Chem Eng J. 2022;430:132924. https://doi.org/10.1016/j.cej.2021.132924.
Sio JEL, Escobar EC, Kim H, Chung WJ, Nisola GM. Hydroxypicolinic acid tethered on magnetite core-silica shell (HPCA@SiO2@Fe3O4) as an effective and reusable adsorbent for practical Co(II) recovery. Chemosphere. 2022;298:134301. https://doi.org/10.1016/j.chemosphere.2022.134301.
Tran TT, Moon HS, Lee MS. Recovery of cobalt, nickel and copper compounds from UHT processed spent lithium-ion batteries by hydrometallurgical process. Min Process Extract Metall Rev 2021; https://doi.org/10.1080/08827508.2021.1910508.
Li JL, Lu YQ, Yang TR, Ge DY, Wood DL, Li Z. Water-based electrode manufacturing and direct recycling of lithium-ion battery electrodes-a green and sustainable manufacturing system. iScience. 2020;23(5):101081. https://doi.org/10.1016/j.isci.2020.101081.
Zheng XH, Zhu ZW, Lin X, Zhang Y, He Y, Cao HB, Sun Z. A mini-review on metal recycling from spent lithium ion batteries. Engineering. 2018;4(3):361. https://doi.org/10.1016/j.eng.2018.05.018.
Li YK, Lv WG, Huang HL, Yan WY, Li XK, Ning PG, Cao HB, Sun Z. Recycling of spent lithium-ion batteries in view of green chemistry. Green Chem. 2021;23(17):6139. https://doi.org/10.1039/d1gc01639c.
Xiao JF, Guo J, Zhan L, Xu ZM. A cleaner approach to the discharge process of spent lithium ion batteries in different solutions. J Clean Product. 2020;255:120064. https://doi.org/10.1016/j.jclepro.2020.120064.
Yao LP, Zeng Q, Qi T, Li J. An environmentally friendly discharge technology to pretreat spent lithium-ion batteries. J Clean Product. 2020;245:118820. https://doi.org/10.1016/j.jclepro.2019.118820.
Hagelüken C. Recycling of elctronic scrap at Umicore’s integrated metals smelter and refinery. Proc EMC. 2005;59(3):152.
Kim S, Bang J, Yoo J, Shin Y, Bae J, Jeong J, Kim K, Dong P, Kwon K. A comprehensive review on the pretreatment process in lithium-ion battery recycling. J Clean Product. 2021;294:126329. https://doi.org/10.1016/j.jclepro.2021.126329.
Wang MM, Tan QY, Liu LL, Li JH. A facile, environmentally friendly, and low-temperature approach for decomposition of polyvinylidene fluoride from the cathode electrode of spent lithium-ion batteries. ACS Sustain Chem Eng. 2019;7(15):12799. https://doi.org/10.1021/acssuschemeng.9b01546.
Li J, Wang GX, Xu ZM. Generation and detection of metal ions and volatile organic compounds (VOCs) emissions from the pretreatment processes for recycling spent lithium-ion batteries. Waste Manag. 2016;52:221. https://doi.org/10.1016/j.wasman.2016.03.011.
Wang HC. Study on recycling of spent lithium ion batteries containing cobalt and pilot scale experiment. Heilongjiang: Harbin Institute of Technology; 2013.
Zheng RJ. The resynthesis and reuse from materials of spent lithium ion batteries of phosphate and mixture. Heilongjiang: Harbin Institute of Technology; 2017.
Larsson F, Mellander BE. Abuse by external heating, overcharge and short circuiting of commercial lithium-ion battery cells. J Electrochem Soc. 2014;161(10):A1611. https://doi.org/10.1149/2.0311410jes.
Zhang T, He YQ, Wang FF, Ge LH, Zhu XN, Li H. Chemical and process mineralogical characterizations of spent lithium-ion batteries: an approach by multi-analytical techniques. Waste Manage. 2014;34(6):1051. https://doi.org/10.1016/j.wasman.2014.01.002.
Cardarelli. Method for recycling spent lithium metal polymer rechargeable batteries and related materials.US Patent; US 7192564 B2. 2007.
Bruckner L, Frank J, Elwert T. Industrial recycling of lithium-ion batteries-a critical review of metallurgical process routes. Metals. 2020;10(8):1107. https://doi.org/10.3390/met10081107.
Trager T, Friedrich B, Weyhe R. Recovery concept of value metals from automotive lithium-ion batteries. Chem Ing Tec. 2015;87(11):1550. https://doi.org/10.1002/cite.201500066.
Meng K, Xu GY, Peng XH, Youcef-Toumi K, Li J. Intelligent disassembly of electric-vehicle batteries: a forward-looking overview. Resour Conserv Recycl. 2022;182:106207. https://doi.org/10.1016/j.resconrec.2022.106207.
Grützke M, Monnighoff X, Horsthemke F, Kraft V, Winter M, Nowak S. Extraction of lithium-ion battery electrolytes with liquid and supercritical carbon dioxide and additional solvents. RSC Adv. 2015;5(54):43209. https://doi.org/10.1039/c5ra04451k.
Zhao Y, Kang YQ, Fan MC, Li T, Wozny J, Zhou Y, Wang X, Chueh YL, Liang Z, Zhou GM, Wang JX, Tavajohi N, Kang FY, Li BH. Precise separation of spent lithium-ion cells in water without discharging for recycling. Energy Storage Mater. 2022;45:1092. https://doi.org/10.1016/j.ensm.2021.11.005.
Velazquez-Martinez O, Valio J, Santasalo-Aarnio A, Reuter M, Serna-Guerrero R. A critical review of lithium-ion battery recycling processes from a circular economy perspective. Batteries-Basel. 2019;5(4):68. https://doi.org/10.3390/batteries5040068.
Diekmann J, Hanisch C, Frobose L, Schalicke G, Loellhoeffel T, Folster AS, Kwade A. Ecological recycling of lithium-ion batteries from electric vehicles with focus on mechanical processes. J Electrochem Soc. 2017;164(1):A6184. https://doi.org/10.1149/2.0271701jes.
Zhang T, He YQ, Ge LH, Fu RS, Zhang X, Huang YJ. Characteristics of wet and dry crushing methods in the recycling process of spent lithium-ion batteries. J Power Sources. 2013;240:766. https://doi.org/10.1016/j.jpowsour.2013.05.009.
Piatek J, Afyon S, Budnyak TM, Budnyk S, Sipponen M, Slabon A. Sustainable Li-ion batteries: chemistry and recycling. Adv Energy Mater. 2021;11(43):2003456. https://doi.org/10.1002/aenm.202003456.
Hossain R, Kumar U, Sahajwalla V. Selective thermal transformation of value added cobalt from spent lithium-ion batteries. J Clean Product. 2021;293:126140. https://doi.org/10.1016/j.jclepro.2021.126140.
Pinegar H, Smith YR. Recycling of end-of-life lithium ion batteries, part I: commercial processes. J Sustain Metall. 2019;5(3):402. https://doi.org/10.1007/s40831-019-00235-9.
Tedjar F, Foudraz JC. Method for the mixed recycling of lithium-based anode batteries and cells. US Patent; US 7820317 B2. 2010.
Pindar S, Dhawan N. Recycling of mixed discarded lithium-ion batteries via microwave processing route. Sustain Mater Technol. 2020;25:e00157. https://doi.org/10.1016/j.susmat.2020.e00157.
Granata G, Pagnanelli F, Moscardini E, Takacova Z, Havlik T, Toro L. Simultaneous recycling of nickel metal hydride, lithium ion and primary lithium batteries: accomplishment of European guidelines by optimizing mechanical pre-treatment and solvent extraction operations. J Power Sour. 2012;212:205. https://doi.org/10.1016/j.jpowsour.2012.04.016.
da Costa A, Matos J, Bernardes A, Muller I. Beneficiation of cobalt, copper and aluminum from wasted lithium-ion batteries by mechanical processing. Int J Miner Process. 2015;145:77. https://doi.org/10.1016/j.minpro.2015.06.015.
Huang Z, Lin M, Qiu RJ, Zhu J, Ruan JJ, Qiu RL. A novel technology of recovering magnetic micro particles from spent lithium-ion batteries by ultrasonic dispersion and waterflow-magnetic separation. Resour Conserv Recycl. 2021;164:105172. https://doi.org/10.1016/j.resconrec.2020.105172.
Cao YX, Wang ZQ, Wang JJ, Li GF. Multi-stage electrostatic separation for recovering of aluminum from fine granules of black dross. J Wuhan Univ Technol-Mater Sci Ed. 2019;34(4):925. https://doi.org/10.1007/s11595-019-2139-2.
Silveira A, Santana M, Tanabe E, Bertuol D. Recovery of valuable materials from spent lithium ion batteries using electrostatic separation. Int J Miner Process. 2017;169:91. https://doi.org/10.1016/j.minpro.2017.11.003.
Bi HJ, Zhu HB, Zu L, Gao Y, Gao S, Bai YX. Environment-friendly technology for recovering cathode materials from spent lithium iron phosphate batteries. Waste Manage Res. 2020;38(8):911. https://doi.org/10.1177/0734242x20931933.
Fan MC, Zhao Y, Kang YQ, Wozny J, Liang Z, Wang JX, Zhou GM, Li BH, Tavajohi N, Kang FY. Room-temperature extraction of individual elements from charged spent LiFePO4 batteries. Rare Met. 2022;41(5):1595. https://doi.org/10.1007/s12598-021-01919-6.
Gratz E, Sa QN, Apelian D, Wang Y. A closed loop process for recycling spent lithium ion batteries. J Power Sour. 2014;262:255. https://doi.org/10.1016/j.jpowsour.2014.03.126.
Vanderbruggen A, Salces A, Ferreira A, Rudolph M, Serna-Guerrero R. Improving separation efficiency in end-of-life lithium-ion batteries flotation using attrition pre-treatment. Minerals. 2022;12(1):72. https://doi.org/10.3390/min12010072.
Saneie R, Abdollahi H, Ghassa S, Azizi D, Chehreh CS. Recovery of copper and aluminum from spent lithium-ion batteries by froth flotation: a sustainable approach. J Sustain Metall. 2022;8(1):386. https://doi.org/10.1007/s40831-022-00493-0.
Zhan RT, Yang ZZ, Bloom I, Pan L. Significance of a solid electrolyte interphase on separation of anode and cathode materials from spent Li-ion batteries by froth flotation. Acs Sustain Chem Eng. 2021;9(1):531. https://doi.org/10.1021/acssuschemeng.0c07965.
Wang FF, Zhang T, He YQ, Zhao YM, Wang S, Zhang GW, Zhang Y, Feng Y. Recovery of valuable materials from spent lithium-ion batteries by mechanical separation and thermal treatment. J Clean Prod. 2018;185:646. https://doi.org/10.1016/j.jclepro.2018.03.069.
Zhang GW, He YQ, Feng Y, Wang HF, Zhu XN. Pyrolysis-ultrasonic-assisted flotation technology for recovering graphite and LiCoO2 from spent lithium-ion batteries. Acs Sustain Chem Eng. 2018;6(8):10896. https://doi.org/10.1021/acssuschemeng.8b02186.
Yu JD, He YQ, Li H, Xie WN, Zhang T. Effect of the secondary product of semi-solid phase Fenton on the flotability of electrode material from spent lithium-ion battery. Powder Technol. 2017;315:139. https://doi.org/10.1016/j.powtec.2017.03.050.
He YQ, Zhang T, Wang FF, Zhang GW, Zhang WG, Wang J. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by Fenton reagent-assisted flotation. J Clean Prod. 2017;143:319. https://doi.org/10.1016/j.jclepro.2016.12.106.
Huang Z, Zhu J, Wu XW, Qiu RJ, Xu ZM, Ruan JJ. Eddy current separation can be used in separation of non-ferrous particles from crushed waste printed circuit boards. J Clean Product. 2021;312:127755. https://doi.org/10.1016/j.jclepro.2021.127755.
Ruan JJ, Dong LP, Zheng J, Zhang T, Huang MZ, Xu ZM. Key factors of eddy current separation for recovering aluminum from crushed e-waste. Waste Manage. 2017;60:84. https://doi.org/10.1016/j.wasman.2016.08.018.
Smith YR, Nagel JR, Rajamani RK. Eddy current separation for recovery of non-ferrous metallic particles: a comprehensive review. Miner Eng. 2019;133:149. https://doi.org/10.1016/j.mineng.2018.12.025.
Bi HJ, Zhu HB, Zu L, Bai YX, Gao S, Gao Y. A new model of trajectory in eddy current separation for recovering spent lithium iron phosphate batteries. Waste Manage. 2019;100:1. https://doi.org/10.1016/j.wasman.2019.08.041.
Liang A. Recycling of spent lithium-ion batteries. Braunschweig: Springer Cham; 2019.
Zhong XH, Liu W, Han JW, Jiao F, Zhu HL, Qin WQ. Pneumatic separation for crushed spent lithium-ion batteries. Waste Manag. 2020;118:331. https://doi.org/10.1016/j.wasman.2020.08.053.
Zhang GW, Du ZX, He YQ, Wang HF, Xie WN, Zhang T. A sustainable process for the recovery of anode and cathode materials derived from spent lithium-ion batteries. Sustainability. 2019;11(8):2363. https://doi.org/10.3390/su11082363.
Bi HJ, Zhu HB, Zu L, He SH, Gao Y, Gao S. Pneumatic separation and recycling of anode and cathode materials from spent lithium iron phosphate batteries. Waste Manag Res. 2019;37(4):374. https://doi.org/10.1177/0734242x18823939.
Shi Y, Zhang J, Bruck AM, Zhang YM, Li J, Stach EA, Takeuchi KJ, Marschilok AC, Takeuchi ES, Yu G. A tunable 3D nanostructured conductive gel framework electrode for high-performance lithium ion batteries. Adv Mater. 2017;29(22):1603922. https://doi.org/10.1002/adma.201603922.
Zhong XH, Han JW, Chen LL, Liu W, Jiao F, Zhu HL, Qin WQ. Binding mechanisms of PVDF in lithium ion batteries. Appl Surf Sci. 2021. https://doi.org/10.1016/j.apsusc.2021.149564.
Nayaka GP, Zhang YJ, Dong P, Wang D, Zhou ZR, Duan JG, Li X, Lin Y, Meng Q, Pai KV, Manjanna J, Santhosh G. An environmental friendly attempt to recycle the spent Li-ion battery cathode through organic acid leaching. J Environ Chem Eng. 2019;7(1):102854. https://doi.org/10.1016/j.jece.2018.102854.
Nan JM, Han DM, Yang MJ, Cui M, Hou XL. Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydride batteries. Hydrometallurgy. 2006;84(1–2):75. https://doi.org/10.1016/j.hydromet.2006.03.059.
Hu ZL, Zhu NW, Wei XR, Zhang SH, Li F, Wu PX, Chen YJ. Efficient separation of aluminum foil from mixed-type spent lithium-ion power batteries. J Environ Manag. 2021;298:113500. https://doi.org/10.1016/j.jenvman.2021.113500.
Makuza B, Tian QH, Guo XY, Chattopadhyay K, Yu DW. Pyrometallurgical options for recycling spent lithium-ion batteries: a comprehensive review. J Power Sour. 2021;491:229622. https://doi.org/10.1016/j.jpowsour.2021.229622.
Song DW, Wang XQ, Nie HH, Shi H, Wang DG, Guo FX, Shi XX, Zhang LQ. Heat treatment of LiCoO2 recovered from cathode scraps with solvent method. J Power Sour. 2014;249:137. https://doi.org/10.1016/j.jpowsour.2013.10.062.
Fan ES, Yang JB, Huang YX, Lin J, Arshad F, Wu F, Li L, Chen RJ. Leaching mechanisms of recycling valuable metals from spent lithium-ion batteries by a malonic acid-based leaching system. ACS Appl Energy Mater. 2020;3(9):8532. https://doi.org/10.1021/acsaem.0c01166.
Bridgwater AV. Waste incineration and pyrolysis. Resour Recov Conserv. 1980. https://doi.org/10.1016/0304-3967(80)90025-6.
Hanisch C, Loellhoeffel T, Diekmann J, Markley KJ, Haselrieder W, Kwade A. Recycling of lithium-ion batteries: a novel method to separate coating and foil of electrodes. J Clean Prod. 2015;108:301. https://doi.org/10.1016/j.jclepro.2015.08.026.
Haldar SK. Chapter 13 - Mineral processing, Edited by S. K. Haldar. Kolkata: Elsevier, Mineral Exploration (Second Edition). 2018:259.
Bernardes AM, Espinosa DCR, Tenório JAS. Recycling of batteries: a review of current processes and technologies. J Power Sour. 2004;130(1–2):291. https://doi.org/10.1016/j.jpowsour.2003.12.026.
Liu W, Zhong XH, Han JW, Qin WQ, Liu T, Zhao CX, Chang ZY. Kinetic study and pyrolysis behaviors of spent LiFePO4 batteries. Acs Sustain Chem Eng. 2019;7(1):1289. https://doi.org/10.1021/acssuschemeng.8b04939.
Tang YQ, Xie HW, Zhang BL, Chen X, Zhao ZQ, Qu JK, Xing PF, Yin HY. Recovery and regeneration of LiCoO2-based spent lithium-ion batteries by a carbothermic reduction vacuum pyrolysis approach: Controlling the recovery of CoO or Co. Waste Management. 2019; 97:140. https://doi.org/10.1016/j.wasman.2019.08.004.
Yang Y, Huang GY, Xu SM, He YH, Liu X. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries. Hydrometallurgy. 2016;165:390. https://doi.org/10.1016/j.hydromet.2015.09.025.
Deng LP, Xu ZJ, Wang MM, Shentu HJ, Liu X, Xiong JW, Cheng YJ, Wang C, Zhou MJ, Gao J, Xia YG. CO2 treatment enables non-hazardous, reliable, and efficacious recovery of spent Li(Ni0.5Co0.2Mn0.3)O2 cathodes. Green Chem. 2022;24(2):779. https://doi.org/10.1039/d1gc02628c.
Tao R, Xing P, Li HQ, Cun ZG, Sun ZH, Wu YF. In situ reduction of cathode material by organics and anode graphite without additive to recycle spent electric vehicle LiMn2O4 batteries. J Power Sour. 2022;520:230827. https://doi.org/10.1016/j.jpowsour.2021.230827.
Xi XL, Feng M, Zhang LW, Nie ZR. Applications of molten salt and progress of molten salt electrolysis in secondary metal resource recovery. Int J Miner Metall Mater. 2020;27(12):1599. https://doi.org/10.1007/s12613-020-2175-0.
Uhlir J. Chemistry and technology of molten salt reactors - history and perspectives. J Nucl Mater. 2007;360(1):6. https://doi.org/10.1016/j.jnucmat.2006.08.008.
Wang MM, Tan QY, Liu LL, Li JH. Efficient separation of aluminum foil and cathode materials from spent lithium-ion batteries using a low-temperature molten salt. ACS Sustain Chem Eng. 2019;7(9):8287. https://doi.org/10.1021/acssuschemeng.8b06694.
Yao ZT, Li JH, Zhao XY. Molten salt oxidation: a versatile and promising technology for the destruction of organic-containing wastes. Chemosphere. 2011;84(9):1167. https://doi.org/10.1016/j.chemosphere.2011.05.061.
Zhao ZL, Li HB, Chen Y, Wu YM. Study on gasification characteristics of waste printed circuit boards (PCB) in molten salts. Acta Sci Circum. 2008;6:1161. https://doi.org/10.13671/j.hjkxxb.2008.06.020.
Xu Q, Wang Y, Shi XY, Zhong YJ, Wu ZG, Song Y, Wang GK, Liu YX, Zhong BH, Guo XD. The direct application of spent graphite as a functional interlayer with enhanced polysulfide trapping and catalytic performance for Li-S batteries. Green Chem. 2021;23(2):942. https://doi.org/10.1039/d0gc04033a.
Divya ML, Natarajan S, Lee YS, Aravindan V. Achieving high-energy dual carbon Li-ion capacitors with unique low- and high-temperature performance from spent Li-ion batteries. J Mater Chem A. 2020;8(9):4950. https://doi.org/10.1039/c9ta13913c.
Yuan MH, Wang HJ, Li HH, Yuan CL, Wang T, Yang HB. Deep eutectic solvent-a novel additive to induce gamma crystallization and alpha-to-gamma phase transition of PVDF. Macromol Chem Phys. 2022;223(4):2100416. https://doi.org/10.1002/macp.202100416.
Wang MM, Tan QY, Liu LL, Li JH. A low-toxicity and high-efficiency deep eutectic solvent for the separation of aluminum foil and cathode materials from spent lithium-ion batteries. J Hazar Mater. 2019;380:120846. https://doi.org/10.1016/j.jhazmat.2019.120846.
Chen XP, Li SZ, Wang Y, Jiang YZ, Tan X, Han WJ, Wang SB. Recycling of LiFePO4 cathode materials from spent lithium-ion batteries through ultrasound-assisted Fenton reaction and lithium compensation. Waste Manag. 2021;136:67. https://doi.org/10.1016/j.wasman.2021.09.026.
Wang MM, Tan QY, Liu LL, Li JH. Revealing the dissolution mechanism of polyvinylidene fluoride of spent lithium-ion batteries in waste oil-based methyl ester solvent. ACS Sustain Chem Eng. 2020;8(19):7489. https://doi.org/10.1021/acssuschemeng.0c02072.
Song X, Hu T, Liang C, Long HL, Zhou L, Song W, You L, Wu ZS, Liu JW. Direct regeneration of cathode materials from spent lithium iron phosphate batteries using a solid phase sintering method. RSC Adv. 2017;7(8):4783. https://doi.org/10.1039/c6ra27210j.
Xu YA, Song DW, Li L, An CH, Wang YJ, Jiao LF, Yuan HT. A simple solvent method for the recovery of LixCoO2 and its applications in alkaline rechargeable batteries. J Power Sour. 2014;252:286. https://doi.org/10.1016/j.jpowsour.2013.11.052.
He K, Zhang ZY, Zhang FS. Synthesis of graphene and recovery of lithium from lithiated graphite of spent Li-ion battery. Waste Manag. 2021;124:283. https://doi.org/10.1016/j.wasman.2021.01.017.
Jiao CY, Sun L, Shao Q, Song JY, Hu Q, Naik N, Guo ZH. Advances in waterborne acrylic resins: synthesis principle, modification strategies, and their applications. ACS Omega. 2021;6(4):2443. https://doi.org/10.1021/acsomega.0c05593.
Nguyen VNH, Lee MS. Separation of Co(II), Ni(II), Mn(II) and Li(I) from synthetic sulfuric acid leaching solution of spent lithium ion batteries by solvent extraction. J Chem Technol Biotechnol. 2021;96(5):1205. https://doi.org/10.1002/jctb.6632.
Pham TP, Cho CW, Yun YS. Environmental fate and toxicity of ionic liquids: a review. Water Res. 2010;44(2):352. https://doi.org/10.1016/j.watres.2009.09.030.
Zheng HS, Huang JQ, Dong T, Sha YF, Zhang HT, Gao J, Zhang SJ. A novel strategy of lithium recycling from spent lithium-ion batteries using imidazolium ionic liquid. Chin J Chem Eng. 2022;41:246. https://doi.org/10.1016/j.cjche.2021.09.020.
Zeng XL, Li JH. Innovative application of ionic liquid to separate Al and cathode materials from spent high-power lithium-ion batteries. J Hazard Mater. 2014;271:50. https://doi.org/10.1016/j.jhazmat.2014.02.001.
Tang SJ, Zhang M, Guo M. A novel deep-eutectic solvent with strong coordination ability and low viscosity for efficient extraction of valuable metals from spent lithium-ion batteries. Acs Sustain Chem Eng. 2022;10(2):975. https://doi.org/10.1021/acssuschemeng.1c06902.
Wang SB, Zhang ZT, Lu ZG, Xu ZH. A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries. Green Chem. 2020;22(14):4473. https://doi.org/10.1039/d0gc00701c.
Wang K, Hu TY, Shi PH, Min YL, Wu JF, Xu QJ. Efficient recovery of value metals from spent lithium-ion batteries by combining deep eutectic solvents and coextraction. Acs Sustain Chem Eng. 2022;10(3):1149. https://doi.org/10.1021/acssuschemeng.1c06381.
Sekharan TR, Chandira RM, Tamilvanan S, Rajesh SC, Venkateswarlu BS. Deep eutectic solvents as an alternate to other harmful solvents. Biointerface Res Appl Chem. 2022;12(1):847. https://doi.org/10.33263/BRIAC121.847860.
Liu CY, Yan QB, Zhang XW, Lei LC, Xiao CL. Efficient recovery of end-of-life NdFeB permanent magnets by selective leaching with deep eutectic solvents. Environ Sci Technol. 2020;54(16):10370. https://doi.org/10.1021/acs.est.0c03278.
Pant D, Dolker T. Green and facile method for the recovery of spent lithium nickel manganese cobalt oxide (NMC) based lithium ion batteries. Waste Manag. 2017;60:689. https://doi.org/10.1016/j.wasman.2016.09.039.
Zhang XH, Xie YB, Cao HB, Nawaz F, Zhang Y. A novel process for recycling and resynthesizing LiNi1/3Co1/3Mn1/3O2 from the cathode scraps intended for lithium-ion batteries. Waste Manag. 2014;34(9):1715. https://doi.org/10.1016/j.wasman.2014.05.023.
Mu DY, Liu Z, Jin S, Liu YL, Tian S, Dai CS. The recovery and recycling of cathode materials and electrolyte from spent lithium ion batteries in full process. Prog Chem. 2020;32(7):950. https://doi.org/10.7536/PC191106.
Liu K, Zhang FS. Innovative leaching of cobalt and lithium from spent lithium-ion batteries and simultaneous dechlorination of polyvinyl chloride in subcritical water. J Hazard Mater. 2016;316:19. https://doi.org/10.1016/j.jhazmat.2016.04.080.
Fu YP, Schuster J, Petranikova M, Ebin B. Innovative recycling of organic binders from electric vehicle lithium-ion batteries by supercritical carbon dioxide extraction. Resour Conserv Recycl. 2021;172:105666. https://doi.org/10.1016/j.resconrec.2021.105666.
Pavon S, Kaiser D, Mende R, Bertau M. The cool-process-a selective approach for recycling lithium batteries. Metals. 2021;11(2):259. https://doi.org/10.3390/met11020259.
Tufail A, Price WE, Hai FI. A critical review on advanced oxidation processes for the removal of trace organic contaminants: a voyage from individual to integrated processes. Chemosphere. 2020;260:127460. https://doi.org/10.1016/j.chemosphere.2020.127460.
Yan SX, Jiang YZ, Chen XP, Zhou T. Improved advanced oxidation process for in situ recycling of Al foils and cathode materials from spent lithium-ion batteries. Ind Eng Chem Res. 2022;61(34):12728. https://doi.org/10.1021/acs.iecr.2c01286.
Amelia D, Karamah EF, Mahardika M, Syafri E, Rangappa SM, Siengchin S, Asrofi M. Effect of advanced oxidation process for chemical structure changes of polyethylene microplastics. Mater Today-Proc. 2022;52:2501. https://doi.org/10.1016/j.matpr.2021.10.438.
Miao F, Liu YF, Gao MM, Yu X, Xiao PW, Wang M, Wang SG, Wang XH. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J Hazard Mater. 2020;399:123023. https://doi.org/10.1016/j.jhazmat.2020.123023.
Chen XP, Li SZ, Wu X, Zhou T, Ma HR. In-situ recycling of coating materials and Al foils from spent lithium ion batteries by ultrasonic-assisted acid scrubbing. J Clean Product. 2020;258:120943. https://doi.org/10.1016/j.jclepro.2020.120943.
Kayakool FA, Gangaja B, Nair S, Santhanagopalan D. Li-based all-carbon dual-ion batteries using graphite recycled from spent Li-ion batteries. Sustain Mater Technol. 2021;28:00262. https://doi.org/10.1016/j.susmat.2021.e00262.
Wang HF, Liu JS, Bai XJ, Wang S, Yang D, Fu YP, He YQ. Separation of the cathode materials from the Al foil in spent lithium-ion batteries by cryogenic grinding. Waste Manag. 2019;91:89. https://doi.org/10.1016/j.wasman.2019.04.058.
Liu JS, Wang HF, Hu TT, Bai XJ, Wang S, Xie WN, Hao J, He YQ. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by cryogenic grinding and froth flotation. Miner Eng. 2020;148:106223. https://doi.org/10.1016/j.mineng.2020.106223.
Liang SB, Hao YC. A novel cryogenic grinding system for recycling scrap tire peels. Adv Powder Technol. 2000;11(2):187. https://doi.org/10.1163/156855200750172303.
Liu Y, Yu HJ, Wang Y, Tang D, Qiu WX, Li WZ, Li J. Microwave hydrothermal renovating and reassembling spent lithium cobalt oxide for lithium-ion battery. Waste Manage. 2022;143:186. https://doi.org/10.1016/j.wasman.2022.02.024.
Usman M, Cheema SA, Farooq M. Heterogeneous Fenton and persulfate oxidation for treatment of landfill leachate: a review supplement. J Clean Product. 2020;256:120448. https://doi.org/10.1016/j.jclepro.2020.120448.
Chao YW, Liu BG, Zhang H, Tian SH, Zhang LB, Guo SH, Zhou BC. Efficient recovery and regeneration of waste graphite through microwave stripping from spent batteries anode for high-performance lithium-ion batteries. J Clean Product. 2022;333:130197. https://doi.org/10.1016/j.jclepro.2021.130197.
Fan WW, Zhang JL, Ma RX, Chen YQ, Wang CY. Regeneration of graphite anode from spent lithium-ion batteries via microwave calcination. J Electroanal Chem. 2022;908:116087. https://doi.org/10.1016/j.jelechem.2022.116087.
Fu YP, He YQ, Yang Y, Qu LL, Li JL, Zhou R. Microwave reduction enhanced leaching of valuable metals from spent lithium -ion batteries. J Alloy Compd. 2020;832:154920. https://doi.org/10.1016/j.jallcom.2020.154920.
Haccuria E, Crivits T, Hayes PC, Jak E. Selected phase equilibria studies in the Al2O3-CaO-SiO2 system. J Am Ceram Soc. 2016;99(2):691. https://doi.org/10.1111/jace.13991.
He K, Zhang ZY, Alai L, Zhang FS. A green process for exfoliating electrode materials and simultaneously extracting electrolyte from spent lithium-ion batteries. J Hazard Mater. 2019;375:43. https://doi.org/10.1016/j.jhazmat.2019.03.120.
Grützke M, Kraft V, Weber W, Wendt C, Friesen A, Klamor S, Winter M, Nowak S. Supercritical carbon dioxide extraction of lithium-ion battery electrolytes. J Supercrit Fluids. 2014;94:216. https://doi.org/10.1016/j.supflu.2014.07.014.
Sun L, Qiu KQ. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J Hazard Mater. 2011;194:378. https://doi.org/10.1016/j.jhazmat.2011.07.114.
Lei SY, Sun W, Yang Y. Solvent extraction for recycling of spent lithium-ion batteries. J Hazard Mater. 2022;424:127654. https://doi.org/10.1016/j.jhazmat.2021.127654.
Tong DG, Lai QY, Ji XY. Recycling of LiCoO2 cathode materials from spent lithium ion batteries. J Chem Ind Eng. 2005;56(10):1967.
Fomo G, Madzimbamuto TN, Ojumu TV. Applications of nonconventional green extraction technologies in process industries: challenges, limitations and perspectives. Sustainability. 2020;12(13):5244. https://doi.org/10.3390/su12135244.
Buszewski B, Wrona O, Mayya RP, Zakharenko AM, Kalenik TK, Golokhvast KS, Piekoszewski W, Rafińska K. The potential application of supercritical CO2 in microbial inactivation of food raw materials and products. Critic Rev Food Sci Nutr. 2021;62(24):6535. https://doi.org/10.1080/10408398.2021.1902939.
Liu YL, Mu DY, Li RH, Ma QX, Zheng RJ, Dai CS. Purification and characterization of reclaimed electrolytes from spent lithium-ion batteries. J Phys Chem C. 2017;121(8):4181. https://doi.org/10.1021/acs.jpcc.6b12970.
Rothermel S, Evertz M, Kasnatscheew J, Qi X, Gruetzke M, Winter M, Nowak S. Graphite recycling from spent lithium-ion batteries. Chemsuschem. 2016;9(24):3473. https://doi.org/10.1002/cssc.201601062.
Jung S, Kwon D, Park S, Kwon K, Tsang YF, Kwon EE. Valorization of a spent lithium-ion battery electrolyte through syngas formation using CO2-assisted catalytic thermolysis over a battery cathode material. J CO2 Utilization. 2021;50: 101591. https://doi.org/10.1016/j.jcou.2021.101591.
Zhong XH, Liu W, Han JW, Jiao F, Qin WQ, Liu T, Zhao CX. Pyrolysis and physical separation for the recovery of spent LiFePO4 batteries. Waste Manag. 2019;89:83. https://doi.org/10.1016/j.wasman.2019.03.068.
Yi XX, Qi YP, Li FF, Shu JC, Sun Z, Sun SH, Chen MJ, Pu SY. Effect of electrolyte reuse on metal recovery from waste CPU slots by slurry electrolysis. Waste Manag. 2019;95:370. https://doi.org/10.1016/j.wasman.2019.06.034.
Umicore recycling division home page. 2022. https://www.umicore.cn/ (Accessed Aug 1, 2022).
Mayyas A, Steward D, Mann M. The case for recycling: overview and challenges in the material supply chain for automotive li-ion batteries. Sustain Mater Technol. 2019;19:e00087. https://doi.org/10.1016/j.susmat.2018.e00087.
Retriev technologies home page. 2022. https://www.retrievtech.com/ (Accessed Aug 1, 2022).
Jin S, Mu DY, Lu Z, Li RH, Liu Z, Wang Y, Tian S, Dai CS. A comprehensive review on the recycling of spent lithium-ion batteries: urgent status and technology advances. J Clean Product. 2022;340:130535. https://doi.org/10.1016/j.jclepro.2022.130535.
Sonoc A, Jeswiet J, Soo VK. Opportunities to improve recycling of automotive lithium ion batteries. 22nd cirp conference on life cycle engineering. 2015;29:752. https://doi.org/10.1016/j.procir.2015.02.039.
Accurec battery recycling division home page. 2022. https://accurec.de/geschichte (2022.8.1).
Recupyl battery recycling division home page. 2022. www.recupyl.com (2022.8.1).
Batrec home page. 2022. https://batrec.ch/en/ (2022.8.1).
Hanisch C, Diekmann J, Stieger A, Haselrieder W, Kwade A. Recycling of lithium-ion batteries. In handbook of clean energy systems. 2015:1. https://doi.org/10.1002/9781118991978.hces221.
AkkuSer Oy Home page. 2022. https://www.akkuser.fi/en/home/ (2022.8.1).
Green eco-manufacturer home page. 2022. http://www.gemchina.com/ (2022.8.1).
Brunp home page. 2022. https://www.brunp.com.cn/ (2022.8.1).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 52074177 and 52174391) and Hunan Provincial Science and Technology Plan, China (No. 2017TP1001). The authors also appreciate the editor(s) and anonymous reviewer(s) with gratitude for their professional comments and constructive suggestions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g., a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, SX., Jiang, YZ., Chen, XP. et al. Engineering classification recycling of spent lithium-ion batteries through pretreatment: a comprehensive review from laboratory to scale-up application. Rare Met. 43, 915–941 (2024). https://doi.org/10.1007/s12598-023-02377-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02377-y