Abstract
The increasing demand for clean energy and growing concerns regarding environmental sustainability have led to greater attention devoted toward the production of clean fuels via green chemistry. In this respect, ammonia is a green alternative to fossil fuels and can serve as a clean energy source. There is now great interest in realizing the electrochemical reduction in atmospheric nitrogen (N2) for cheap, environmentally friendly and reliable ammonia (NH3) production worldwide. However, the robustness of the triple bond in N2 and the low efficiency of candidate catalysts limit the utility of this conversion. Single atom catalysts have been found to be more effective than nanoparticles due to their unique properties, and thus have been studied extensively for the nitrogen reduction reaction. In this review, we have covered the recent advances in design and synthesis of noble metal and non-noble metal single atom catalysts for the electrochemical reduction in nitrogen during the years 2018–2022. The catalyst efficiencies, with reference to coordination preferences and theoretical studies have been discussed. Moreover, we also provide insights into the current challenges and some considerations for further future studies.
摘要
随着人们对清洁能源需求的增加, 以及对环境可持续性的担忧日益加剧, 通过绿色化学生产清洁燃料受到越来越多的关注。氨是化石燃料的绿色替代品, 可以作为清洁能源。目前, 人们对实现大气中氮气 (N2) 的电化学还原以生产氨 (NH3), 从而在全球范围内实现廉价、环保和可靠的氨生产产生了浓厚兴趣。然而, N2中三键的稳定性以及催化剂的低效率限制了这一转化的实用性。单原子催化剂因其独特的性质而比纳米催化剂更高效, 并已被广泛应用于氮还原反应 (NRR) 。在这篇综述中, 我们介绍了2018 − 2022年以来用于电化学氮还原的贵金属和非贵金属基单原子催化剂 (SAC) 的设计和合成的最新进展。基于对配位环境和理论计算的研究, 对催化剂的效率进行了讨论。此外, 还在与NRR的进一步研究相关的当前挑战和未来前景方面提出了我们的见解。
Graphical abstract



Reproduced with permission from Ref. [45]. Copyright 2019, the Royal Society of Chemistry

Reproduced with permission from Ref. [47]. Copyright 2016, American Chemical Society

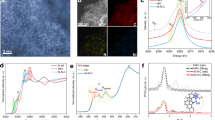
Reproduced with permission from Ref. [60]. Copyright 2015, Elsevier. b Comparison of HER and NRR limiting-potential volcanoes. HER and NRR overpotentials (UL) as a function of *N binding-energy (ΔGN) descriptor are shown in blue and black, respectively, and individual metal points are labeled for (111) (left) and (211) (right) surfaces. Here from the graph, it is clear that HER limiting potentials are consistently less negative than those for NRR. Reproduced with permission from Ref. [61]. Copyright 2018, American Chemical Society

Reproduced with permission from Ref. [69]. Copyright 2013, Springer Nature. b Schematic illustration of thermally stable Pd catalyst synthesized using ALD. Redrawn from Ref. [70]. Copyright 2016, the Royal Society of Chemistry. c Schematic illustration of metal SACs on graphene oxide (GO). Reproduced with permission from Ref. [71]. Copyright 2018, Springer Nature. d Schematic diagram of synthesis of Ni/graphdiyne and Fe/graphdiyne (GD). Reproduced with permission from Ref. [73]. Copyright 2018, Nature Publishing Group. e Deposition of iridium species. Panels (1) and (2) show cathodic and anodic electrochemical deposition mechanism, respectively; iridium mass loadings as a function of iridium concentration in 1 mol·L−1 KOH electrolyte for (3) cathodic and (4) anodic deposition. Cycle number of scanning was set at 10 and 3 for cathodic and anodic deposition, respectively. Reproduced with permission from Ref. [74]. Copyright 2020, Nature Publishing Group


Reproduced with permission from Ref. [27]. Copyright 2019, WILEY–VCH Verlag GmbH & Co. KGaA, Weinheim

Reproduced with permission from Ref. [117]. Copyright 2019, American Chemical Society

Reproduced with permission from Ref. [121]. Copyright 2019, WILEY–VCH Verlag GmbH & Co. KGaA, Weinheim
Similar content being viewed by others
References
Wan J, Zheng J, Zhang H, Wu A, Li X. Single atom catalysis for electrocatalytic ammonia synthesis. Catal Sci Technol. 2022;12(1):38. https://doi.org/10.1039/D1CY01442K.
Hao Q, Liu C, Jia G, Wang Y, Arandiyan H, Wei W, Ni BJ. Catalytic reduction of nitrogen to produce ammonia by bismuth-based catalysts: state of the art and future prospects. Mater Horiz. 2020;7(4):1014. https://doi.org/10.1039/C9MH01668F.
Schlögl R. Catalytic synthesis of ammonia—a “never-ending story”? Angew Chem Int Ed. 2003;42(18):2004. https://doi.org/10.1002/anie.200301553.
Chen GF, Ren S, Zhang L, Cheng H, Luo Y, Zhu K, Ding LX, Wang H. Nitrogen reduction reactions: advances in electrocatalytic N2 reduction—strategies to tackle the selectivity challenge. Small Methods. 2019;3(6):1970016. https://doi.org/10.1002/smtd.20.
Shen H, Yang M, Hao L, Wang J, Strunk J, Sun Z. Photocatalytic nitrogen reduction to ammonia: insights into the role of defect engineering in photocatalysts. Nano Res. 2022;15:2773. https://doi.org/10.1007/s12274-021-3725-0.
Hu P, Huang Z, Amghouz Z, Makkee M, Xu F, Kapteijn F, Dikhtiarenko A, Chen Y, Gu X, Tang XF. Electronic metal-support interactions in single-atom catalysts. Angew Chem. 2014;126(13):3486. https://doi.org/10.1002/anie.201309248.
Liu JD, Wei ZX, Dou YH, Feng YZ, Ma JM. Ru-doped phosphorene for electrochemical ammonia synthesis. Rare Met. 2020;39(8):874. https://doi.org/10.1007/s12598-020-01451-z.
Légaré MA, Bélanger-Chabot G, Dewhurst RD, Welz E, Krummenacher I, Engels B, Braunschweig H. Nitrogen fixation and reduction at boron. Science. 2018;359(6378):896. https://doi.org/10.1126/science.aaq1684.
McCoy D, Feo T, Harvey T, Prum R. Structural absorption by barbule microstructures of super black bird of paradise feathers. Nat Commun. 2018;9:1. https://doi.org/10.1038/s41467-017-02088-w.
Tang C, Qiao SZ. How to explore ambient electrocatalytic nitrogen reduction reliably and insightfully. Chem Soc Rev. 2019;48(12):3166. https://doi.org/10.1039/C9CS00280D.
Chen Y, Wang L, Yao Z, Hao L, Tan X, Masa J, Robertson AW, Sun Z. Tuning the coordination structure of single atoms and their interaction with the support for carbon dioxide electroreduction. Acta Phys Chim Sin. 2022;38(11):2206020. https://doi.org/10.3866/PKU.WHXB202207024.
Gao Y, Yang Y, Hao L, Hong S, Tan X, Wu TS, Sun Z. Single Nb atom modified anatase TiO2 (110) for efficient electrocatalytic nitrogen reduction reaction. Chem Catal. 2022. https://doi.org/10.1016/j.checat.2022.06.010.
Jiang Y, Sung Y, Choi C, Joo Bang G, Hong S, Tan X, Wu TS, Soo YL, Xiong P, Li MJ, Hao L, Jung Y, Sun Z. Single-atom molybdenum-N3 sites for selective hydrogenation of CO2 to CO. Angew Chem Int Ed. 2022;61:202203836. https://doi.org/10.1002/anie.202203836.
Jia M, Fan Q, Liu S, Qiu J, Sun Z. Single-atom catalysis for electrochemical CO2 reduction. Curr Opin Green Sustain Chem. 2019;16:1. https://doi.org/10.1016/j.cogsc.2018.11.002.
Yao Y, Wang J, Shahid UB, Gu M, Wang H, Li H, Shao MJ. Electrochemical synthesis of ammonia from nitrogen under mild conditions: current status and challenges. Electrochem Energy Rev. 2020;3(2):239. https://doi.org/10.1007/s41918-019-00061-3.
Shi L, Yin Y, Wang S, Sun H. Rational catalyst design for N2 reduction under ambient conditions: strategies toward enhanced conversion efficiency. ACS Catal. 2020;10(12):6870. https://doi.org/10.1021/acscatal.0c01081.
Li M, Huang H, Low J, Gao C, Long R, Xiong YJ. Recent progress on electrocatalyst and photocatalyst design for nitrogen reduction. Small Methods. 2019;3(6):1800388. https://doi.org/10.1002/smtd.201800388.
Li Y, Zhang Q, Li C, Fan HN, Luo WB, Liu HK, Dou SX. Atomically dispersed metal dimer species with selective catalytic activity for nitrogen electrochemical reduction. J Mater Chem A. 2019;7(39):22242. https://doi.org/10.1039/C9TA07845B.
Lazouski N, Chung M, Williams K, Gala ML, Manthiram K. Non-aqueous gas diffusion electrodes for rapid ammonia synthesis from nitrogen and water-splitting-derived hydrogen. Nat Catal. 2020;3(5):463. https://doi.org/10.1038/s41929-020-0455-8.
Liu Y, Xu Q, Fan X, Quan X, Su Y, Chen S, Yu H, Cai Z. Electrochemical reduction of N2 to ammonia on Co single atom embedded N-doped porous carbon under ambient conditions. J Mater Chem A. 2019;7(46):26358. https://doi.org/10.1039/C9TA10382A.
Zang W, Yang T, Zou H, Xi S, Zhang H, Liu X, Kou Z, Du Y, Feng YP, Shen L. Copper single atoms anchored in porous nitrogen-doped carbon as efficient pH-universal catalysts for the nitrogen reduction reaction. ACS Catal. 2019;9(11):10166. https://doi.org/10.1021/acscatal.9b02944.
Gao Y, Han Z, Hong S, Wu T, Li X, Qiu J, Sun Z. ZIF-67-derived cobalt/nitrogen-doped carbon composites for efficient electrocatalytic N2 reduction. ACS Appl Energy Mater. 2019;2(8):6071. https://doi.org/10.1021/acsaem.9b01135.
Lv F, Zhao S, Guo R, He J, Peng X, Bao H, Fu J, Han L, Qi G, Luo J. Nitrogen-coordinated single Fe sites for efficient electrocatalytic N2 fixation in neutral media. Nano Energy. 2019;61:420. https://doi.org/10.1016/j.nanoen.2019.04.092.
Wang M, Liu S, Qian T, Liu J, Zhou J, Ji H, Xiong J, Zhong J, Yan C. Over 56.55% Faradaic efficiency of ambient ammonia synthesis enabled by positively shifting the reaction potential. Nat Commun. 2019;10(1):341. https://doi.org/10.1038/s41467-018-08120-x.
Wang Y, Cui X, Zhao J, Jia G, Gu L, Zhang Q, Meng L, Shi Z, Zheng L, Wang C. Rational design of Fe-N/C hybrid for enhanced nitrogen reduction electrocatalysis under ambient conditions in aqueous solution. ACS Catal. 2018;9(1):336. https://doi.org/10.1021/acscatal.8b03802.
Zhang R, Jiao L, Yang W, Wan G, Jiang HL. Single-atom catalysts templated by metal-organic frameworks for electrochemical nitrogen reduction. J Mater Chem A. 2019;7(46):26371. https://doi.org/10.1039/C9TA10206J.
Han L, Liu X, Chen J, Lin R, Liu H, Lü F, Bak S, Liang Z, Zhao S, Stavitski EJ. Atomically dispersed molybdenum catalysts for efficient ambient nitrogen fixation. Angew Chem Int Ed. 2019;58(8):2321. https://doi.org/10.1002/anie.201811728.
Yuan LP, Wu ZY, Jiang WJ, Tang T, Niu S, Hu JS. Phosphorus-doping activates carbon nanotubes for efficient electroreduction of nitrogen to ammonia. Nano Res. 2020;13(5):1376. https://doi.org/10.1007/s12274-020-2637-8.
Tao H, Choi C, Ding LX, Jiang Z, Han Z, Jia M, Fan Q, Gao Y, Wang H, Robertson A. Nitrogen fixation by Ru single-atom electrocatalytic reduction. Chem. 2019;5(1):204. https://doi.org/10.1016/j.chempr.2018.10.007.
Yu B, Li H, White J, Donne S, Yi J, Xi S, Fu Y, Henkelman G, Yu H, Chen ZJ. Tuning the catalytic preference of ruthenium catalysts for nitrogen reduction by atomic dispersion. Adv Funct Mater. 2019;30(6):1905665. https://doi.org/10.1002/adfm.201905665.
Liu J, Kong X, Zheng L, Guo X, Liu X, Shui J. Rare earth single-atom catalysts for nitrogen and carbon dioxide reduction. ACS Nano. 2020;14(1):1093. https://doi.org/10.1021/acsnano.9b08835.
Wang Q, Zheng G, Hao S, Liu X, Zheng J, Wang Y, Su Z, Xu N, He Y, Lei L, Zhang X. Au1Co1 alloy supported on graphene oxide with enhanced performance for ambient electrolysis of nitrogen to ammonia. ACS Sustain Chem Eng. 2020;8(1):44. https://doi.org/10.1021/acssuschemeng.9b06827.
Zhang L, Ji X, Ren X, Luo Y, Shi X, Asiri AM, Zheng B, Sun X. Correction to “Efficient electrochemical N2 reduction to NH3 on MoN nanosheets array under ambient conditions.” ACS Sustain Chem Eng. 2018;7(1):1807. https://doi.org/10.1021/acssuschemeng.8b05648.
Wu X, Wang Z, Han Y, Zhang D, Wang M, Li H, Zhao H, Pan Y, Lai J, Wang L. Chemically coupled NiCoS/C nanocages as efficient electrocatalysts for nitrogen reduction reactions. J Mater Chem A. 2020;8(2):543. https://doi.org/10.1039/C9TA10142J.
Liu Y, Huang L, Zhu X, Fang Y, Dong S. Coupling Cu with Au for enhanced electrocatalytic activity of nitrogen reduction reaction. Nanoscale. 2020;12(3):1811. https://doi.org/10.1039/C9NR08788E.
Liu A, Gao M, Ren X, Meng F, Yang Y, Yang Q, Guan W, Gao L, Liang X, Ma T. A two-dimensional Ru@MXene catalyst for highly selective ambient electrocatalytic nitrogen reduction. Nanoscale. 2020;12(20):10933. https://doi.org/10.1039/D0NR00788A.
Zhao R, Liu C, Zhang X, Zhu X, Wei P, Ji L, Guo Y, Gao S, Luo Y, Wang Z. An ultrasmall Ru2P nanoparticles-reduced graphene oxide hybrid: an efficient electrocatalyst for NH3 synthesis under ambient conditions. J Mater Chem A. 2020;8(1):77. https://doi.org/10.1039/C9TA10346E.
Li L, Tang C, Xia B, Jin H, Zheng Y, Qiao S. Two-dimensional mosaic bismuth nanosheets for highly selective ambient electrocatalytic nitrogen reduction. ACS Catal. 2019;9(4):2902. https://doi.org/10.1021/acscatal.9b00366.
Zhang X, Wu T, Wang H, Zhao R, Chen H, Wang T, Wei P, Luo Y, Zhang Y, Sun X. Boron nanosheet: an elemental two-dimensional (2D) material for ambient electrocatalytic N2-to-NH3 fixation in neutral media. ACS Catal. 2019;9(5):4609. https://doi.org/10.1021/acscatal.8b05134.
Wang Z, Gong F, Zhang L, Wang R, Ji L, Liu Q, Luo Y, Guo H, Li Y, Gao P. Electrocatalytic hydrogenation of N2 to NH3 by MnO: experimental and theoretical investigations. Adv Sci. 2018;6(1):1801182. https://doi.org/10.1002/advs.201801182.
Zhang L, Ding L, Chen G, Yang X, Wang H. Ammonia synthesis under ambient conditions: selective electroreduction of dinitrogen to ammonia on black phosphorus nanosheets. Angew Chem. 2019;131(9):2638. https://doi.org/10.1002/anie.201813174.
Zhao J, Wang B, Zhou Q, Wang H, Li X, Chen H, Wei Q, Wu D, Luo Y, You JJ. Efficient electrohydrogenation of N2 to NH3 by oxidized carbon nanotubes under ambient conditions. Chem Comm. 2019;55(34):4997. https://doi.org/10.1039/C9CC00726A.
Zhu X, Wu T, Ji L, Li C, Wang T, Wen S, Gao S, Shi X, Luo Y, Peng Q. Ambient electrohydrogenation of N2 for NH3 synthesis on non-metal boron phosphide nanoparticles: the critical role of P in boosting the catalytic activity. J Mater Chem A. 2019;7(27):16117. https://doi.org/10.1039/C9TA05016G.
Zhang G, Ji Q, Zhang K, Chen Y, Li Z, Liu H, Li J, Qu J. Triggering surface oxygen vacancies on atomic layered molybdenum dioxide for a low energy consumption path toward nitrogen fixation. Nano Energy. 2019;59:10. https://doi.org/10.1016/j.nanoen.2019.02.028.
Guo W, Zhang K, Liang Z, Zou R, Xu Q. Electrochemical nitrogen fixation and utilization: theories, advanced catalyst materials and system design. Chem Soc Rev. 2019;48(24):5658. https://doi.org/10.1039/C9CS00159J.
Yan X, Liu D, Cao H, Hou F, Liang J, Dou SX. Nitrogen reduction to ammonia on atomic-scale active sites under mild conditions. Small Methods. 2019;3(9):1800501. https://doi.org/10.1002/smtd.201800501.
Li XF, Li QK, Cheng J, Liu L, Yan Q, Wu Y, Zhang XH, Wang ZY, Qiu Q, Luo Y. Conversion of dinitrogen to ammonia by FeN3-embedded graphene. J Am Chem Soc. 2016;138(28):8706. https://doi.org/10.1021/jacs.6b04778.
Shen H, Choi C, Masa J, Li X, Qiu J, Jung Y, Sun Z. Electrochemical ammonia synthesis: mechanistic understanding and catalyst design. Chem. 2021;7(7):1708. https://doi.org/10.1016/j.chempr.2021.01.009.
Ling C, Bai X, Ouyang Y, Du A, Wang J. Single molybdenum atom anchored on N-doped carbon as a promising electrocatalyst for nitrogen reduction into ammonia at ambient conditions. J Phys Chem C. 2018;122(25):16842. https://doi.org/10.1021/acs.jpcc.8b05257.
van der Ham CJM, Koper MTM, Hetterscheid DGH. Challenges in reduction of dinitrogen by proton and electron transfer. Chem Soc Rev. 2014;43(15):5183. https://doi.org/10.1039/C4CS00085D.
Nishibayashi Y. Recent progress in transition-metal-catalyzed reduction of molecular dinitrogen under ambient reaction conditions. Inorg Chem. 2015;54(19):9234. https://doi.org/10.1021/acs.inorgchem.5b00881.
Singh AR, Rohr BA, Schwalbe JA, Cargnello M, Chan K, Jaramillo TF, Chorkendorff I, Nørskov JK. Electrochemical ammonia synthesis-the selectivity challenge. ACS Catal. 2016;7(1):706. https://doi.org/10.1021/acscatal.6b03035.
Wang S, Ichihara F, Pang H, Chen H, Ye J. Nitrogen fixation reaction derived from nanostructured catalytic materials. Adv Funct Mater. 2018;28(50):1803309. https://doi.org/10.1002/adfm.201803309.
Zhou F, Azofra LM, Ali M, Kar M, Simonov AN, McDonnell-Worth C, Sun C, Zhang X, MacFarlane DR. Electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquids. Energy Environ Sci. 2017;10(12):2516. https://doi.org/10.1039/C7EE02716H.
Suryanto BHR, Kang CSM, Wang D, Xiao C, Zhou F, Azofra LM, Cavallo L, Zhang X, MacFarlane D. Rational electrode-electrolyte design for efficient ammonia electrosynthesis under ambient conditions. ACS Energy Lett. 2018;3(6):1219. https://doi.org/10.1021/acsenergylett.8b00487.
Zhang L, Mallikarjun Sharada S, Singh AR, Rohr BA, Su Y, Qiao L, Nørskov J. A theoretical study of the effect of a non-aqueous proton donor on electrochemical ammonia synthesis. Phys Chem Chem Phys. 2018;20(7):4982. https://doi.org/10.1039/C7CP05484J.
Koh CSL, Lee HK, Fan Sim HY, Han X, Phan-Quang GC, Ling XY. Turning water from a hindrance to the promotor of preferential electrochemical nitrogen reduction. Chem Mater. 2020;32(4):1674. https://doi.org/10.1021/acs.chemmater.9b05313.
Zheng J, Lyu Y, Qiao M, Wang R, Zhou Y, Li H, Chen C, Li Y, Zhou H, Wang S. Photoelectrochemical synthesis of ammonia on the aerophilic-hydrophilic heterostructure with 37.8% efficiency. Chem. 2019;5(3):617. https://doi.org/10.1016/j.chempr.2018.12.003.
Ou P, Zhou X, Meng F, Chen C, Chen Y, Song J. Single molybdenum center supported on N-doped black phosphorus as an efficient electrocatalyst for nitrogen fixation. Nanoscale. 2019;11(28):13600. https://doi.org/10.1039/C9NR02586C.
Medford AJ, Vojvodic A, Hummelshøj JS, Voss J, Abild-Pedersen F, Studt F, Bligaard T, Nilsson A, Nørskov J. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J Catal. 2015;328:36. https://doi.org/10.1016/j.jcat.2014.12.033.
Montoya JH, Tsai C, Vojvodic A, Nørskov JK. The challenge of electrochemical ammonia synthesis: a new perspective on the role of nitrogen scaling relations. Chemsuschem. 2015;8(13):2180. https://doi.org/10.1002/cssc.201500322.
Stamenkovic VR, Mun BS, Arenz M, Mayrhofer KJJ, Lucas CA, Wang G, Ross PN, Markovic N. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat Mater. 2007;6(3):241. https://doi.org/10.1038/nmat1840.
Choi C, Back S, Kim NY, Lim J, Kim YH, Jung Y. Suppression of hydrogen evolution reaction in electrochemical N2 reduction using single-atom catalysts: a computational guideline. ACS Catal. 2018;8(8):7517. https://doi.org/10.1021/acscatal.8b00905.
Zhu C, Fu S, Shi Q, Du D, Lin Y. Single-atom electrocatalysts. Angew Chem Int Ed. 2017;56(45):13944. https://doi.org/10.1039/D0RA08223F.
Liu P, Zhao Y, Qin R, Mo S, Chen G, Gu L, Chevrier DM, Zhang P, Guo Q, Zang D. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science. 2016;352(6287):797. https://doi.org/10.1126/science.aaf5251.
Thomas JM. The concept, reality and utility of single-site heterogeneous catalysts (SSHCs). Phys Chem Chem Phys. 2014;16(17):7647. https://doi.org/10.1039/C4CP00513A.
O’Neill BJ, Jackson DHK, Lee J, Canlas C, Stair PC, Marshall CL, Elam J, Kuech T, Dumesic J, Huber G. Catalyst design with atomic layer deposition. ACS Catal. 2015;5(3):1804. https://doi.org/10.1021/cs501862h.
Mohanty B, Jena BK, Basu S. Single atom on the 2D matrix: an emerging electrocatalyst for energy applications. ACS Omega. 2020;5(1):1287. https://doi.org/10.1021/acsomega.9b03515.
Sun S, Zhang G, Gauquelin N, Chen N, Zhou J, Yang S, Chen W, Meng X, Geng D, Banis M. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Sci Rep. 2013;3(1). https://doi.org/10.1038/srep01775.
Piernavieja-Hermida M, Lu Z, White A, Low KB, Wu T, Elam JW, Wu Z, Lei Y. Towards ALD thin film stabilized single-atom Pd1 catalysts. Nanoscale. 2016;8(33):15348. https://doi.org/10.1039/C6NR04403D.
Fei H, Dong J, Feng Y, Allen CS, Wan C, Volosskiy B, Li M, Zhao Z, Wang Y, Sun H. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat Catal. 2018;1(1):63. https://doi.org/10.1038/s41929-017-0008-y.
Choi CH, Kim M, Kwon HC, Cho SJ, Yun S, Kim HT, Mayrhofer KJ, Kim H, Choi MJ. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst. Nat Commun. 2016;7(1):10922. https://doi.org/10.1038/ncomms10922.
Xue Y, Huang B, Yi Y, Guo Y, Zuo Z, Li Y, Jia Z, Liu H, Li Y. Anchoring zero valence single atoms of nickel and iron on graphdiyne for hydrogen evolution. Nat Commun. 2018;9(1):1460. https://doi.org/10.1038/s41467-018-03896-4.
Zhang Z, Feng C, Liu C, Zuo M, Qin L, Yan X, Xing Y, Li H, Si R, Zhou S. Electrochemical deposition as a universal route for fabricating single-atom catalysts. Nat Commun. 2020;11(1):1215. https://doi.org/10.1038/s41467-020-14917-6.
Kwak DH, Han SB, Lee YW, Park HS, Choi IA, Ma KB, Kim MC, Kim SJ, Kim DH, Sohn JI. Fe/N/S-doped mesoporous carbon nanostructures as electrocatalysts for oxygen reduction reaction in acid medium. Appl Catal B Environ. 2017;203:889. https://doi.org/10.1016/j.apcatb.2016.10.084.
Hu K, Tao L, Liu D, Huo J, Wang S. Sulfur-doped Fe/N/C nanosheets as highly efficient electrocatalysts for oxygen reduction reaction. ACS Appl Mater Interfaces. 2016;8(30):19379. https://doi.org/10.1021/acsami.6b02078.
Wang XX, Cullen DA, Pan Y, Hwang S, Wang M, Feng Z, Wang J, Engelhard MH, Zhang H, He Y. Nitrogen-coordinated single cobalt atom catalysts for oxygen reduction in proton exchange membrane fuel cells. Adv Mater. 2018;30(11):1706758. https://doi.org/10.1002/adma.201706758.
Li JC, Wei Z, Liu D, Du D, Lin Y, Shao M. Dispersive single-atom metals anchored on functionalized nanocarbons for electrochemical reactions. Top Curr Chem. 2019. https://doi.org/10.1007/s41061-018-0229-9.
Peng Y, Lu B, Chen S. Carbon-supported single atom catalysts for electrochemical energy conversion and storage. Adv Mater. 2018;30(48):1801995. https://doi.org/10.1002/adma.201801995.
Yin P, Yao T, Wu Y, Zheng L, Lin Y, Liu W, Ju H, Zhu J, Hong X, Deng Z. Single atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew Chem. 2016;128(36):10958. https://doi.org/10.1002/anie.201604802.
Zhang C, Sha J, Fei H, Liu M, Yazdi S, Zhang J, Zhong Q, Zou X, Zhao N, Yu H. Single-atomic ruthenium catalytic sites on nitrogen-doped graphene for oxygen reduction reaction in acidic medium. ACS Nano. 2017;11(7):6930. https://doi.org/10.1021/acsnano.7b02148.
Fei H, Dong J, Arellano-Jiménez MJ, Ye G, Dong Kim N, Samuel ELG, Peng Z, Zhu Z, Qin F, Bao J. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat Commun. 2015;6(1):8668. https://doi.org/10.1038/ncomms9668.
Cui L, Cui L, Li Z, Zhang J, Wang H, Lu S, Xiang Y. A copper single-atom catalyst towards efficient and durable oxygen reduction for fuel cells. J Mater Chem A. 2019;7(28):16690. https://doi.org/10.1039/C9TA03518D.
Li X, Yang X, Huang Y, Zhang T, Liu B. Supported noble-metal single atoms for heterogeneous catalysis. Adv Mater. 2019;31(50):1902031. https://doi.org/10.1002/adma.201902031.
Geng Z, Liu Y, Kong X, Li P, Li K, Liu Z, Du J, Shu M, Si R, Zeng J. Achieving a record-high yield rate of 120.9 μgNH3.mgcat−1·h−1 for N2 electrochemical reduction over Ru single-atom catalysts. Adv Mater. 2018;30(40):1803498. https://doi.org/10.1002/adma.201803498
Yang J, Li W, Wang D, Li Y. Electronic metal-support interaction of single-atom catalysts and applications in electrocatalysis. Adv Mater. 2020;32(49):2003300. https://doi.org/10.1002/adma.202003300.
Chen G, Ding M, Zhang K, Shen Z, Wang Y, Ma J, Wang A, Li Y, Xu H. Single-atomic ruthenium active sites on Ti3C2 MXene with oxygen-terminated surface synchronize enhanced activity and selectivity for electrocatalytic nitrogen reduction to ammonia. Chemsuschem. 2022;15(3): e202102352. https://doi.org/10.1002/cssc.202102352.
Peng W, Luo M, Xu X, Jiang K, Peng M, Chen D, Chan TS, Tan Y. Spontaneous atomic ruthenium doping in Mo2CTX MXene defects enhances electrocatalytic activity for the nitrogen reduction reaction. Adv Energy Mater. 2020;10(25):2001364. https://doi.org/10.1002/aenm.202001364.
Wang X, Wang W, Qiao M, Wu G, Chen W, Yuan T, Xu Q, Chen M, Zhang Y, Wang X. Atomically dispersed Au1 catalyst towards efficient electrochemical synthesis of ammonia. Sci Bull. 2018;63(19):1246. https://doi.org/10.1016/j.scib.2018.07.005.
Qin Q, Heil T, Antonietti M, Oschatz M. Single-site gold catalysts on hierarchical N-doped porous noble carbon for enhanced electrochemical reduction of nitrogen. Small Methods. 2018;2(12):1800202. https://doi.org/10.1002/smtd.201800202.
Guo C, Ran J, Vasileff A, Qiao SZ. Rational design of electrocatalysts and photo(electro)catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions. Energy Environ Sci. 2018;11(1):45. https://doi.org/10.1039/C7EE02220D.
Bao D, Zhang Q, Meng F-L, Zhong HX, Shi MM, Zhang Y, Yan JM, Jiang Q, Zhang X. Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2/NH3 cycle. Adv Mater. 2016;29(3):1604799. https://doi.org/10.1002/adma.201604799.
Shi MM, Bao D, Wulan BR, Li YH, Zhang YF, Yan JM, Jiang Q. Au sub-nanoclusters on TiO2 toward highly efficient and selective electrocatalyst for N2 conversion to NH3 at ambient conditions. Adv Mater. 2017;29(17):1606550. https://doi.org/10.1002/adma.201606550.
Zhao Y, Shi R, Bian X, Zhou C, Zhao Y, Zhang S, Wu F, Waterhouse GI, Wu LZ, Tung C. Ammonia detection methods in photocatalytic and electrocatalytic experiments: how to improve the reliability of NH3 production rates? Adv Sci. 2019;6(8):1802109. https://doi.org/10.1002/advs.201802109.
Chen G, Ren S, Zhang L, Cheng H, Luo Y, Zhu K, Ding LX, Wang H. Advances in electrocatalytic N2 reduction—strategies to tackle the selectivity challenge. Small Methods. 2018;3(6):1800337. https://doi.org/10.1002/smtd.201800337.
Qu Y, Li Z, Chen W, Lin Y, Yuan T, Yang Z, Zhao C, Wang J, Zhao C, Wang X. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms. Nat Catal. 2018;1(10):781. https://doi.org/10.1038/s41929-018-0146-x.
Jin Z, Li P, Fang Z, Yu G. Emerging electrochemical techniques for probing site behavior in single-atom electrocatalysts. Acc Chem Res. 2022;55(5):759. https://doi.org/10.1021/acs.accounts.1c00785.
Wang Y, Chen Z, Han P, Du Y, Gu Z, Xu X, Zheng G. Single-atomic Cu with multiple oxygen vacancies on ceria for electrocatalytic CO2 reduction to CH4. ACS Catal. 2018;8(8):7113. https://doi.org/10.1021/acscatal.8b01014.
Qiu Y, Peng X, Lü F, Mi Y, Zhuo L, Ren J, Liu X, Luo J. Single-atom catalysts for the electrocatalytic reduction of nitrogen to ammonia under ambient conditions. Chem Asian J. 2019;14(16):2770. https://doi.org/10.1002/asia.201900793.
Guo X, Huang S. Tuning nitrogen reduction reaction activity via controllable Fe magnetic moment: a computational study of single Fe atom supported on defective graphene. Electrochim Acta. 2018;284:392. https://doi.org/10.1016/j.electacta.2018.07.168.
Zhang Q, Guan J. Single-atom catalysts for electrocatalytic applications. Adv Funct Mater. 2020;30(31):2000768. https://doi.org/10.1002/adfm.202000768.
Li L, Chang X, Lin X, Zhao ZJ, Gong J. Theoretical insights into single-atom catalysts. Chem Soc Rev. 2020;49(22):8156. https://doi.org/10.1039/D0CS00795A.
Huang J, Zhang Q, Ding J, Zhai Y. Fe-N-C single atom catalysts for the electrochemical conversion of carbon, nitrogen and oxygen elements. Mater Rep Energy. 2020;2(3):100141. https://doi.org/10.1016/j.matre.2022.100141.
Wang Y, Wang D, Li Y. Rational design of single-atom site electrocatalysts: from theoretical understandings to practical applications. Adv Mater. 2021;33(34):2008151. https://doi.org/10.1002/adma.202008151.
Zhang S, Jin M, Shi T, Han M, Sun Q, Lin Y, Ding Z, Zheng LR, Wang G, Zhang Y. Electrocatalytically active Fe-(O-C2)4 single-atom sites for efficient reduction of nitrogen to ammonia. Angew Chem. 2020;132(32):13525. https://doi.org/10.1002/anie.202005930.
Su H, Chen L, Chen Y, Si R, Wu Y, Wu X, Geng Z, Zhang W, Zeng J. Single atoms of iron on MoS2 nanosheets for N2 electroreduction into ammonia. Angew Chem. 2020;132(46):20591. https://doi.org/10.1002/anie.202009217.
Li J, Chen S, Quan F, Zhan G, Jia F, Ai Z, Zhang L. Accelerated dinitrogen electroreduction to ammonia via interfacial polarization triggered by single-atom protrusions. Chem. 2020;6(4):885. https://doi.org/10.1016/j.chempr.2020.01.013.
Mukherjee S, Yang X, Shan W, Samarakoon W, Karakalos S, Cullen DA, More K, Wang M, Feng Z, Wang G. Atomically dispersed single Ni site catalysts for nitrogen reduction toward electrochemical ammonia synthesis using N2 and H2O. Small Methods. 2020;4(6):1900821. https://doi.org/10.1002/smtd.201900821.
Guo X, Wan X, Shui J. Molybdenum-based materials for electrocatalytic nitrogen reduction reaction. Cell Rep Phys Sci. 2021;2(6):100447. https://doi.org/10.1016/j.xcrp.2021.100447.
Zhang C, Wang Z, Lei J, Ma L, Yakobson BI, Tour JM. Atomic molybdenum for synthesis of ammonia with 50% Faradic efficiency. Small. 2022;18(15):2106327. https://doi.org/10.1002/smll.202106327.
Hui L, Xue Y, Yu H, Liu Y, Fang Y, Xing C, Huang B, Li Y. Highly efficient and selective generation of ammonia and hydrogen on a graphdiyne-based catalyst. J Am Chem Soc. 2019;141(27):10677. https://doi.org/10.1021/jacs.9b03004.
Ma Y, Yang T, Zou H, Zang W, Kou Z, Mao L, Feng Y, Shen L. Synergizing Mo single atoms and Mo2C nanoparticles on CNTs synchronizes selectivity and activity of electrocatalytic N2 reduction to ammonia. Adv Mater. 2020;32(33):2002177. https://doi.org/10.1002/adma.202002177.
Wang X, Wu D, Liu S, Zhang J, Fu XZ, Luo JL. Folic acid self-assembly enabling manganese single-atom electrocatalyst for selective nitrogen reduction to ammonia. Nano Micro Lett. 2021;13(1):125. https://doi.org/10.1007/s40820-021-00651-1.
Wei ZX, Zhu YT, Liu JY, Zhang ZC, Hu WP, Xu H, Ma JM. Recent advance in single-atom catalysis. Rare Met. 2021;40(4):767. https://doi.org/10.1007/s12598-020-01592-1.
Wen Y, Zhu H, Hao J, Lu S, Zong W, Lai F, Ma P, Dong W, Liu T, Du M. Metal-free boron and sulphur Co-doped carbon nanofibers with optimized p-band centers for highly efficient nitrogen electroreduction to ammonia. Appl Catal B Environ. 2021;292:120144. https://doi.org/10.1016/j.apcatb.2021.120144.
Yu X, Han P, Wei Z, Huang L, Gu Z, Peng S, Ma J, Zheng G. Boron-doped graphene for electrocatalytic N2 reduction. Joule. 2018;2(8):1610. https://doi.org/10.1016/j.joule.2018.06.007.
Liu C, Li Q, Wu C, Zhang J, Jin Y, MacFarlane DR, Sun C. Single-boron catalysts for nitrogen reduction reaction. J Am Chem Soc. 2019;141(7):2884. https://doi.org/10.1021/jacs.8b13165.
Chen H, Liang X, Liu Y, Ai X, Asefa T, Zou X. Active site engineering in porous electrocatalysts. Adv Mater. 2020;32(44):2002435. https://doi.org/10.1002/adma.202002435.
Yao Y, Zhu S, Wang H, Li H, Shao MA. Spectroscopic study on the nitrogen electrochemical reduction reaction on gold and platinum surfaces. J Am Chem Soc. 2018;140(4):1496. https://doi.org/10.1021/jacs.7b12101.
Yin H, Li SL, Gan LY, Wang P. Pt-embedded in monolayer g-C3N4 as a promising single-atom electrocatalyst for ammonia synthesis. J Mater Chem A. 2019;7(19):11908. https://doi.org/10.1039/C9TA01624D.
Saeidi N, Esrafili MD, Sardroodi JJ. Electrochemical reduction of N2 to NH3 using a Co-atom stabilized on defective N-doped graphene: a computational study. Chem Select. 2019;4(42):12216. https://doi.org/10.1002/slct.201903206.
Huang Y, Yang T, Yang L, Liu R, Zhang G, Jiang J, Luo Y, Lian P, Tang S. Graphene–boron nitride hybrid-supported single Mo atom electrocatalysts for efficient nitrogen reduction reaction. J Mater Chem A. 2019;7(25):15173. https://doi.org/10.1039/C9TA02947H.
Ling C, Ouyang Y, Li Q, Bai X, Mao X, Du A, Wang J. A General two-step strategy-based high-throughput screening of single atom catalysts for nitrogen fixation. Small Methods. 2018;3(9):1800376. https://doi.org/10.1002/smtd.201800376.
He T, Matta SK, Du A. Single tungsten atom supported on N-doped graphyne as a high-performance electrocatalyst for nitrogen fixation under ambient conditions. Phys Chem. 2019;21(3):1546. https://doi.org/10.1039/C8CP06978F.
Bian X, Zhao Y, Zhang S, Li D, Shi R, Zhou C, Zhang T. Enhancing the supply of activated hydrogen to promote photocatalytic nitrogen fixation. ACS Mater Lett. 2021;3(11):1521. https://doi.org/10.1021/acsmaterialslett.1c00504.
Huang H, Shi R, Zhang X, Zhao J, Su C, Zhang T. Photothermal-assisted triphase photocatalysis over a multifunctional bilayer paper. Angew Chem Int Ed. 2021;60(42):22963. https://doi.org/10.1002/anie.202110336.
Zhou P, Chao Y, Lv F, Lai J, Wang K, Guo S. Designing noble metal single-atom-loaded two-dimension photocatalyst for N2 and CO2 reduction via anion vacancy engineering. Sci Bull. 2020;65(9):720. https://doi.org/10.1016/j.scib.2019.12.025.
Zhao Z, Park J, Choi C, Hong S, Hui X, Zhang H, Lo TWB, Robertson AW, Lv Z, Jung Y, Sun Z. Engineering vacancy and hydrophobicity of two-dimensional TaTe2 for efficient and stable electrocatalytic N2 reduction. The Innovation. 2022;3(1): 100190. https://doi.org/10.1016/j.xinn.2021.100190.
Xi J, Jung HS, Xu Y, Xiao F, Bae JW, Wang S. Synthesis strategies, catalytic applications, and performance regulation of single-atom catalysts. Adv Func Mater. 2021;31(12):2008318. https://doi.org/10.1002/adfm.202008318.
Cheng H, Ding LX, Chen GF, Zhang L, Xue J, Wang H. Molybdenum carbide nanodots enable efficient electrocatalytic nitrogen fixation under ambient conditions. Adv Mater. 2018;30(46):1803694. https://doi.org/10.1002/adma.201803694.
Chen ZW, Yan JM, Jiang Q. Single or double: which is the altar of atomic catalysts for nitrogen reduction reaction? Small Methods. 2019;3(6):1800291. https://doi.org/10.1002/smtd.201800291.
Qiu N, Li J, Wang H, Zhang Z. Emerging dual-atomic-site catalysts (DASCs) for electrocatalytic CO2 reduction. Sci China Mater. 2022. https://doi.org/10.1007/s40843-022-2189-x.
Yang C, Zhu Y, Liu J, Qin Y, Wang H, Liu H, Hu W. Defect engineering for electrochemical nitrogen reduction reaction to ammonia. Nano Energy. 2020;77:105126. https://doi.org/10.1016/j.nanoen.2020.105126.
Chen C, Sun M, Wang K, Li Y. Dual-metal single-atomic catalyst: the challenge in synthesis, characterization, and mechanistic investigation for electrocatalysis. SmartMat. 2022. https://doi.org/10.1002/smm2.1085.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21972010) and Beijing Natural Science Foundation (No. 2192039).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iqbal, M.S., Yao, ZB., Ruan, YK. et al. Single-atom catalysts for electrochemical N2 reduction to NH3. Rare Met. 42, 1075–1097 (2023). https://doi.org/10.1007/s12598-022-02215-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02215-7