Abstract
With the development of environmental monitoring, it is urgent to establish NO2 sensor with good sensing performance. Compared with the traditional NO2 sensors made of metal oxides, NO2 sensors made of n-p heterostructure nanocomposites have good sensing performance in detection limit and operating temperature. ZnO nanoflake arrays with polyaniline film grown on the surface were prepared on ceramic tubes by hydrothermal and vapor diffusion method. The gas-phase diffusion method can control the heterostructure by adjusting the diffusion time. At room temperature (25 °C), the construction of rich n-p heterogeneous interface enables the sensor prepared by the nanocomposite to respond to NO2, showing the sensing performance with the response value of 28.00 to 10.00 × 10–6 NO2; the detection limit improved to 0.01 × 10–6 and the recovery time of 18 s. In this work, the sensing mechanism of NO2 at heterogeneous interface is analyzed, which provides a promising material for the detection of low concentration NO2 at room temperature.
Graphical abstract

摘要
随着环境监测需求的不断增加, 研制性能优异的二氧化氮传感器已迫在眉睫。与传统的金属氧化物NO2传感器相比, 具有n-p异质结构的纳米复合材料将在检测限和工作温度方面显示出更大的优势。本文采用水热法在陶瓷管上原位生长ZnO纳米片阵列, 并利用气相扩散法在ZnO阵列表面覆盖聚苯胺, 得到复合物薄膜, 并考察了聚苯胺扩散时间对气敏性能的影响。丰富n-p异质界面的ZnO/PANI复合材料传感器能够对NO2响应, 对10.00 × 10-6浓度的NO2响应值为28.00, 检测限为0.01 × 10-6, 恢复时间18 s。研究了NO2的敏感机理, 能在室温条件下检测低浓度NO2使ZnO/PANI成为气敏传感器的理想材料。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
NO2 is one of the main harmful air pollutants with pungent smell, which can cause acid rain, haze weather and photochemical smog, posing a great threat to ecosystem and human health [1,2,3,4]. According to the regulations of the American Conference of Governmental Industrial Hygienists, the safety standard concentration of NO2 in environmental monitoring is 3 × 10–6 [5]. The United States Environmental Protection Agency (US EPA) also announced that the exposure concentration of NO2 in the air is 0.053 × 10–6 [6]. People’s health can be affected if people stay in the environment with NO2 concentration greater than 3 × 10–6 for more than 8 h, or greater than 5 × 10–6 for more than 15 min. In addition, the detection of NO2 concentration in exhaled gas can be used as a monitoring index for some diseases [7]. Therefore, the development of NO2 gas sensors is indispensable for environmental monitoring and medical diagnosis.
Zinc oxide (ZnO) is regarded as one of the potential materials for detecting NO2 owing to its excellent conductivity and stability [8]. Most traditional metal oxide sensors are thick film sensors (usually several microns in thickness), which makes it difficult for the bottom layer to fully take part in the whole gas sensing process [9]. Therefore, thin-film metal oxide arrays with highly ordered morphology can provide more active sites because of the larger specific surface area. For example, ZnO nanorod arrays with different lengths grown in situ by wet chemical method have 3100% gas sensing response to 100 × 10–6 NO2 at 175 °C [10]. The vertically aligned ZnO nanowalls prepared on indium tin oxides (ITO) glass with zinc acetate as zinc source have a response value of 30 to 50 × 10–6 NO2 at 220 °C and a detection limit of 1 × 10–6 [11]. However, ZnO sensors usually have the disadvantage of high operating temperature, which lead to increased energy consumption, complex device structure and easy to cause explosion [12, 13]. In addition, the detection of low concentration NO2 is still a challenge for ZnO sensor.
Conductive polymers have been proved to effectively reduce the operating temperature of gas sensing materials, including polyaniline (PANI), polypyrrole (PPy), polythiophene (PTh) and their derivatives [14,15,16,17,18]. PANI has become an ideal material due to its high conductivity and low cost. In particular, PANI has a large specific surface area, which can promote the adsorption and desorption of gas, so as to shorten the response and recovery time [19, 20]. However, there are few reports on sensors prepared by ZnO/PANI nanocomposite for NO2 detection. For instance, after preparing ZnO-TiO2 nanoparticles, Sonker et al. [21] spin coated a layer of PANI film to make the sensor capable of detecting liquefied petroleum gas (LPG) and NO2. The response value to 20 × 10–6 NO2 at room temperature is 412. Xu et al. [22] synthesized ZnO/SnO2 porous nanoparticles by solvothermal method, and the surface of the nanoparticles was coated with PANI. The response/recovery time of ZnO/SnO2/PANI nanocomposite (9 s/27 s) is shorter than that of ZnO/SnO2 nanocomposite (28 s/20 s). However, the detection limit is only 2.5 × 10–6 NO2. The combination of PANI not only improves the response value, but also reduces the response recovery time. However, the detection limit and recovery time need to be further improved for popularization and practical application. Therefore, the diffusion of PANI on the surface of ZnO nanoflake array is expected to realize the detection of trace NO2 at room temperature.
Our research team [23] previously reported that forest-like PANI/TiO2 nanorod arrays can detect low concentrations of dimethylamine at room temperature. Compared with the traditional composed method, the gas-phase diffusion method can control the heterostructure by adjusting the diffusion time. ZnO/PANI nanocomposites show the properties of n-type semiconductor and have superior sensing performance to NO2 at room temperature. Compared with the original ZnO and PANI, the sensing performance of ZnO/PANI nanocomposite sensor has been significantly improved. The response value is about 23 times higher than that of original PANI and 2 times higher than that of original ZnO. The detection limit is improved to 0.01 × 10–6, which is the best detection limit of all ZnO/PANI-based NO2 sensors ever reported in the literature.
2 Experimental
Zinc acetate (Zn(Ac)·2H2O), diethanolamine (DEA), hydrochloric acid (HCl), ethanol (C2H5OH), hexamethylene tetramine (C6H12N4), ammonium persulfate (APS), and aniline (C6H7N) all directly purchased of analytical grade reagents without further purification. Characterization, sensor fabrication and sensing measurements are described in the Supporting Information.
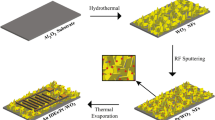
For the preparation of ZnO/PANI nanoflake arrays, first of all, 0.1147 g APS was dissolved in 20 ml deionized water by stirring. Then, ceramic tubes and glass slides with ZnO nanoflake arrays (kindly see the Supporting Information) were immersed in the above solution. After holding for 120 s, the ceramic tube was pulled out of the liquid surface with a dip-coating machine at a speed of 35 mm·min−1 and hung on the top of the desiccator (Φ15 cm). Subsequently, 100 μl aniline was placed at the bottom of the desiccator. ZnO/PANI nanoflake arrays composites were obtained after aniline volatilized at 1 °C for 0.5 h. Finally, ZnO/PANI composites were washed with deionized water, and dried in an oven at 70 °C for 12 h. Figure 1 shows the schematic diagram of synthesis processes of ZnO/PANI nanocomposites thin film.
3 Results and discussion
3.1 Morphology, structural and surface chemical characterization
X-ray diffraction (XRD, D/max-iii-b-40 kv, Japan, with Cu Kα1 radiation, λ = 0.15406 nm) patterns of PANI, ZnO and ZnO/PANI nanocomposites are shown in Fig. 2a. The significant peak from 20° to 30° is attributed to PANI [24]. The diffraction peaks at 31.77°, 34.42°, 62.86°, 36.25° and 56.60° correspond to (100), (002), (103), (101) and (110) crystal planes of ZnO (JCPDS No. 36-1451). Therefore, the nanocomposite is composed of ZnO and PANI.
In order to further verify the structure of the nanocomposites and the existence of PANI, the nanocomposites were studied by Fourier transform infrared spectroscopy (FTIR). Figure 2b shows FTIR spectra of ZnO, PANI and ZnO/PANI nanocomposites in the wave number range of 500–4000 cm−1. The peak at 564 cm−1 is caused by the tensile vibration of Zn–O belonging to ZnO. The peaks at 1577, 1499, 1297, 1107 and 1025 cm−1 are mainly attributed to PANI. The peak at 1577 cm−1 belongs to C=C stretching of quinoid rings, 1499 cm−1 belongs to C=C stretching of benzenoid rings, and 1297 cm−1 belongs to the C–N stretching of secondary aromatic amine [25,26,27,28]. The peak at 1107 cm−1 is designated as the protonated imine. The peak at 1025 cm−1 represents the tension of C–H bending. A slight blue shift of PANI peaks can be observed from the nanocomposite, which is due to the interactions between ZnO and PANI [29].
The morphology and microstructure of the composite were characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Uniform ZnO nanoflake arrays can be observed on the ceramic tube (Fig. 3a, b). As shown in SEM images of ZnO/PANI nanocomposites (Fig. 3c, d), PANI nanoflakes are of 10–20 nm thickness and 1–2 μm in diameter.
In order to better observe the microstructure of ZnO/PANI nanocomposite, TEM was used to characterize the material. It is obvious that PANI is evenly distributed on the surface of smooth ZnO nanoflakes (Fig. 4a, b), which is the same as that obtained by SEM. High-resolution transmission electron microscope (HRTEM) clearly shows the interface between ZnO and PANI (Fig. 4c). 0.24 nm is the characteristic plane spacing corresponding to (101) plane of ZnO. The selected area electron diffraction (SAED) shows the diffraction lattice corresponding to (104), (103), (100) and (006) crystal planes of ZnO (Fig. 4d), which further confirms XRD results.
The chemical state and element composition of ZnO/PANI nanocomposite were studied by X-ray photoelectron spectroscopy (XPS) (Fig. S1 in Supporting Information). According to the full-range XPS spectrum and binding energy, there are C, N, O and Zn in ZnO/PANI nanocomposite. The analysis shows that C and N come from PANI, which indicates that PANI has been successfully modified on the surface of ZnO.
3.2 NO2 gas sensing properties
The diffusion time of aniline directly affects the effective heterogeneous interface between PANI and ZnO. Therefore, Fig. 5a displays the effect of aniline diffusion time in the range of 0.5–2.5 h on the response value of the sensor prepared by the material. As ZnO/PANI nanocomposites sensor reflects the nature of n-type semiconductor, the response value is defined as S = Rg/Ra, where Ra is the resistance in the presence of air and Rg is the resistance after exposure to NO2 gas. The response of the sensor to 10.00 × 10–6 NO2 reaches the maximum of 28.00, when the diffusion time is 0.5 h. When the diffusion time is greater than 0.5 h, the response value decreases. The possible reason is that after a long time of deposition, ZnO is wrapped by PANI, which reduces the number of exposed heterogeneous interfaces and the adsorption sites of NO2, thus reducing the response value. Therefore, the samples prepared in 0.5 h were selected for study in detail.
Figure 5b depicts the response values of ZnO, PANI and ZnO/PANI to 10.00 × 10–6 NO2 by histogram. At the operating temperature of 50 °C, the response value of ZnO sensor was 14.00. At room temperature, the PANI sensor response value was 1.19. ZnO/PANI sensor achieved the high response of 28.00 at room temperature, which is 23.53 times higher than that of PANI sensor and 2 times higher than that of ZnO sensor. This result indicates that the formed heterojunction can effectively improve the response value. More interestingly, ZnO/PANI reflects the sensing characteristics of n-type semiconductor. The results are similar to those previously reported by our group. The reason is that a very thin PANI film is formed on the surface of ZnO by gas diffusion method. Although this film has a large effective coverage area on ZnO, it is not completely covered. The combination of PANI and ZnO enhances its electrical conductivity, making ZnO sensor can work at room temperature. The array structure of ZnO provides more active sites, which is conducive to the diffusion, adsorption of test gas molecules and the transmission of electrons. Therefore, ZnO plays a leading role. The excellent selectivity determines that the sensor can accurately detect NO2 without being affected by other gases. When evaluating the selectivity of ZnO/PANI sensor, it was found that the response values to 100 × 10–6 ethanol, acetone, hydrogen sulfide, triethylamine, trimethylamine, aniline, dry ammonia, and dimethyl amine (for the detailed preparation procedures for all sample gases, kindly see the Supporting Information Sensor Manufacturing and Sensing Measurements section) were 1.00, 1.00, 4.66, 1.57, 1.07, 1.30, 1.84 and 2.27, respectively (Fig. 5c). It proves that ZnO/PANI sensor has excellent selectivity for NO2.
Figure 6a presents the response and recovery curve of the sensor at room temperature. When the sensor was exposed to 10.00 × 10–6 NO2, the response value of the sensor was 28.00. The sensor response and recovery time were 32 and 18 s, respectively. The 18 s recovery time can be attributed to the highly ordered nanoflake array structure, which is favorable for the desorption of NO2 molecules on the material.
Gas sensing properties of ZnO/PANI sensor at 25% relative humidity at room temperature: a one-cycle resistance signal to 10.00 × 10–6 NO2; b reproducibility of response to 10.00 × 10–6 NO2 for five times; c response test results of 0.01 × 10–6–10.00 × 10–6 NO2; d linear relationship between concentration and response value; e long-term stability evaluation of 1.00 × 10–6 NO2; f sensor response value at 11.30% to 67.00% relative humidity
Figure 6b shows the response and recovery curves for five times when the sensor was exposed to 10.00 × 10–6 NO2 at room temperature. The response values of five tests were 28.00, 27.56, 28.00, 28.36 and 27.65, respectively. This demonstrates that the monitoring of NO2 by ZnO/PANI sensor has excellent repeatability at room temperature. Figure 6c shows the response test results of the sensor to NO2 with the concentration between 0.01 × 10–6 and 10.00 × 10–6. As shown in Fig. 6d, there is a good corresponding relationship between the response value (y) and the concentration. A1, A2, x0 and dx represent the initial value, final value, center and time const, respectively. The correlation coefficient (R2) is as high as 0.99874. The detection limit is as low as 0.01 × 10–6 (Rg/Ra = 1.17). The data indicate that the sensor has a wide detection range.
Figure 6e shows the response of the sensor to 1.00 × 10–6 NO2 every 6 days in a month. The average response value was 1.91. It shows that ZnO/PANI sensor has good long-term stability. The response values of ZnO/PANI sensor under different humidity environments are shown in Fig. 6f. When the sensor is exposed to the environment RH 11% (relative humidity) to RH 67%, the response value of ZnO/PANI sensor changes little. The result shows that the sensor can work in normal humidity environment. Comparing the performance of the sensor with the previously reported ZnO-based sensors for detecting NO2 listed in Table 1, it is found that the sensor has significant advantages in operating temperature and detection limit when detecting NO2.
3.3 NO2 sensing mechanism
To investigate the sensing mechanism of NO2, the contact potential difference (CPD) data of PANI and ZnO were tested by Kelvin probe technique (Fig. 7a, b). Evac, Ec, and Ev represent the vacuum band, conduction band and valence band, respectively. The work functions (φ) of PANI and ZnO are 5.27 and 5.47 eV, respectively. The band gap values (Eg) of PANI and ZnO are 2.80 and 3.37 eV [39], respectively. Their carriers exist in the form of holes and electrons. Figure 7c, d shows the schematic diagrams of PANI and ZnO energy bands before and after composite based on work function and band gap. Fermi level of PANI is 0.2 eV higher than that of ZnO. Therefore, electrons will be transferred from PANI conduction band with high Fermi level to ZnO conduction band, and holes will also be transferred from ZnO to PANI, so as to accelerate electron transfer. Electron and hole transfer when the two materials contact each other. Eventually Fermi levels (Ef) at the heterogeneous interface of the ZnO/PANI nanocomposite will become equal. The hole accumulation layer and the electron accumulation layer are created on PANI and ZnO surface, respectively. This results in the decrease in active electrons on the surface of PANI and the increase in active electrons on the surface of ZnO.
When the sensor is exposed to air, oxygen is adsorbed by the composite (Eq. (1)) and combined with the conduction band electrons of the material to form O2− (Eq. (2)) on the surface of the nanocomposite [40, 41]. On the other hand, NO2 is an oxidizing gas [42]. When NO2 molecules are adsorbed on the surface of ZnO/PANI nanocomposite, NO2 can directly capture electrons from the conductive layer of the nanocomposite and convert them into NO2− (Eqs. (3, 4)). NO2 and O2− absorbed by the material are converted to NO2− and O− (Eq. (5)). These lead to an increase in the thickness of the electron depletion layer, resulting in an increase in resistance [43, 44]. When ZnO/PANI sensor leaves NO2 environment, NO2− loses electrons and desorbs from the surface of the nanocomposite to NO2 (Eq. (6)), thereby gradually restoring the resistance in air.
From the performance test and mechanism analysis, it can be seen that the nanocomposite has good response value at room temperature. It is mainly attributed to two factors: One reason is that the heterogeneous interface formed by PANI and ZnO has high activity, which provides active sites for the adsorption of NO2 on the material. Another reason is that the excellent electron transport ability of PANI enables the electron migration caused by NO2 adsorption to be realized.
4 Conclusion
In summary, ZnO/PANI nanoflake arrays were synthesized on ceramic tubes by a combination of hydrothermal method and vapor phase diffusion method. The gas-phase diffusion method can effectively control the heterostructure by adjusting the PANI diffusion time. The combination of PANI reduces the material resistance and realizes room temperature detection. Abundant n-p heterostructures of the sensor improve the sensing performance to NO2. The response value of the ZnO/PANI sensor to 10.00 × 10–6 NO2 at room temperature is 28.00, the response and recovery time is 32 and 18 s, respectively, and the detection limit is down to 0.01 × 10–6. The improvement of electron transport ability and NO2 gas adsorption ability can be attributed to the large number of active sites provided by the nanosheet array structure and heterostructures. The method of preparing the nanocomposite provides the possibility for the detection of many metal oxides at room temperature and has broad application prospects.
References
Das A, Dost R, Richardson T, Grell M, Morrison JJ, Turner ML. A nitrogen dioxide sensor based on an organic transistor constructed from amorphous semiconducting polymers. Adv Mater. 2007;19(22):4018. https://doi.org/10.1002/adma.200701504.
Cui MS, Zhang YQ, Zhong Q, Long ZQ, Zhao N, Huang XW. Portable XRF analysis of noble metal contents for automotive catalysts. Chin J Rare Met. 2020;44(11):1227. https://doi.org/10.13373/j.cnki.cjrm.XY180900111.
Pan QN, Yang ZM, Wang WW, Zhang DZ. Sulfur dioxide gas sensing at room temperature based on tin selenium/tin dioxide hybrid prepared via hydrothermal and surface oxidation treatment. Rare Met. 2021;40(6):1588. https://doi.org/10.1007/s12598-020-01575-2.
Dai ZF, Lee CS, Tian YH, Kim ID, Lee JH. Highly reversible switching from p- to n-type NO2 sensing in a monolayer Fe2O3 inverse opal film and the associated p-n transition phase diagram. J Mater Chem A. 2015;3(7):3372. https://doi.org/10.1039/c4ta05438e.
Shen YB, Wang W, Chen XX, Zhang BQ, Wei DZ, Gao SL, Cui BY. Nitrogen dioxide sensing using tungsten oxide microspheres with hierarchical nanorod-assembled architectures by a complexing surfactant-mediated hydrothermal route. J Mater Chem A. 2016;4(4):1345. https://doi.org/10.1039/c5ta08170j.
Pham T, Li GH, Bekyarova E, Itkis ME, Mulchandani A. MoS2-based optoelectronic gas sensor with sub-parts-per-billion limit of NO2 gas detection. ACS Nano. 2019;13(3):3196. https://doi.org/10.1021/acsnano.8b08778.
Feng QX, Huang BY, Li XG. Graphene-based heterostructure composite sensing materials for detection of nitrogen-containing harmful gases. Adv Funct Mater. 2021;31(41):2104058. https://doi.org/10.1002/adfm.202104058.
Kim JW, Porte Y, Ko KY, Kim H, Myoung JM. Micropatternable double-faced ZnO nanoflowers for flexible gas sensor. ACS Appl Mater Interfaces. 2017;9(38):32876. https://doi.org/10.1021/acsami.7b09251.
Wang MS, Wang YW, Li XJ, Ge CX, Hussain S, Liu GW, Qiao GJ. WO3 porous nanosheet arrays with enhanced low temperature NO2 gas sensing performance. Sens Actuators B Chem. 2020;316:128050. https://doi.org/10.1016/j.snb.2020.128050.
Vanalakar SA, Patil VL, Harale NS, Vhanalakar SA, Gang MG, Jin YK, Patil PS, Kim JH. Controlled growth of ZnO nanorod arrays via wet chemical route for NO2 gas sensor applications. Sens Actuators B Chem. 2015;221:1195. https://doi.org/10.1016/j.snb.2015.07.084.
Yu LM, Wei JS, Luo YY, Tao YL, Lei M, Fan XH, Yan W, Peng P. Dependence of Al3+ on the growth mechanism of vertical standing ZnO nanowalls and their NO2 gas sensing properties. Sens Actuator B Chem. 2014;204:96. https://doi.org/10.1016/j.snb.2014.07.071.
Su YJ, Xie GZ, Tai HL, Li SD, Yang BX, Wang S, Zhang QP, Du HF, Zhang HL, Du XS, Jiang YD. Self-powered room temperature NO2 detection driven by triboelectric nanogenerator under UV illumination. Nano Energy. 2018;47:316. https://doi.org/10.1016/j.nanoen.2018.02.031.
Li ZJ, Li H, Wu ZL, Wang MK, Luo JT, Torun HD, Hu PA, Yang C, Grundman M, Liu XT, Fu YQ. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater Horiz. 2019;6(3):470. https://doi.org/10.1039/c8mh01365a.
Zhang YX, Kim JJ, Chen D, Tuller HL, Rutledge GC. Electrospun polyaniline fibers as highly sensitive room temperature chemiresistive sensors for ammonia and nitrogen dioxide gases. Adv Funct Mater. 2014;24(25):4005. https://doi.org/10.1002/adfm.201400185.
Liu BH, Liu XY, Yuan Z, Jiang YD, Su YJ, Ma JY, Tai HL. A flexible NO2 gas sensor based on polypyrrole/nitrogen-doped multiwall carbon nanotube operating at room temperature. Sens Actuators B Chem. 2019;295:86. https://doi.org/10.1016/j.snb.2019.05.065.
Kamble DB, Sharma AK, Yadav JB, Patil VB, Devan RS, Jatratkar AA, Yewale MA, Ganbavle VV, Pawar SD. Facile chemical bath deposition method for interconnected nanofibrous polythiophene thin films and their use for highly efficient room temperature NO2 sensor application. Sens Actuators B Chem. 2017;244:522. https://doi.org/10.1016/j.snb.2017.01.021.
Dunst K, Karczewski J, Jasiński P. Nitrogen dioxide sensing properties of PEDOT polymer films. Sens Actuators B Chem. 2017;247:108. https://doi.org/10.1016/j.snb.2017.03.003.
Wang DY, Zhang DZ, Guo JY, Hu YQ, Yang Y, Sun TH, Zhang H, Liu XH. Multifunctional poly(vinyl alcohol)/Ag nanofibers-based triboelectric nanogenerator for self-powered MXene/tungsten oxide nanohybrid NO2 gas sensor. Nano Energy. 2021;89:106410. https://doi.org/10.1016/j.nanoen.2021.106410.
Xu HY, Ju DX, Li WR, Gong HB, Zhang J, Wang JQ, Cao BQ. Low-working-temperature, fast-response-speed NO2 sensor with nanoporous SnO2/polyaniline double-layered film. Sens Actuators B Chem. 2016;224:654. https://doi.org/10.1016/j.snb.2015.10.076.
Betty CA, Choudhury S, Arora S. Tin oxide-polyaniline heterostructure sensors for highly sensitive and selective detection of toxic gases at room temperature. Sens Actuators B Chem. 2015;220:288. https://doi.org/10.1016/j.snb.2015.05.074.
Sonker RK, Yadav BC, Gupta V, Tomar M. Fabrication and characterization of ZnO-TiO2-PANI (ZTP) micro/nanoballs for the detection of flammable and toxic gases. J Hazard Mater. 2019;370:126. https://doi.org/10.1016/j.jhazmat.2018.10.016.
Xu HY, Chen XQ, Zhang J, Wang JQ, Cao BQ, Cui DL. NO2 gas sensing with SnO2-ZnO/PANI composite thick film fabricated from porous nanosolid. Sens Actuators B Chem. 2013;176:166. https://doi.org/10.1016/j.snb.2012.09.060.
Yang M, Zhang XF, Guo CY, Cheng XL, Zhu CH, Xu YM, Major Z, Huo LH. Resistive room temperature DMA gas sensor based on the forest-like unusual n-type PANI/TiO2 nanocomposites. Sens Actuators B Chem. 2021;342:130067. https://doi.org/10.1016/j.snb.2021.130067.
Bandgar DK, Navale ST, Vanalkar SA, Kim JH, Harale NS, Patil PS, Patil VB. Synthesis, structural, morphological, compositional and electrical transport properties of polyaniline/α-Fe2O3 hybrid nanocomposites. Synth Met. 2014;195:350. https://doi.org/10.1016/j.synthmet.2014.07.005.
Zhang WY, Zhang XP, Wu ZF, Abdurahman K, Cao YL, Duan HM, Jia DZ. Mechanical, electromagnetic shielding and gas sensing properties of flexible cotton fiber/polyaniline composites. Compos Sci Technol. 2020;188:107966. https://doi.org/10.1016/j.compscitech.2019.107966.
Kim SG, Jun J, Lee JS, Jang J. A highly sensitive wireless nitrogen dioxide gas sensor based on an organic conductive nanocomposite paste. J Mater Chem. 2019;7(14):8451. https://doi.org/10.1039/c9ta00198k.
Bandgar DK, Navale ST, Navale AT, Gupta SK, Aswal DK, Patil VB. Ammonia sensing properties of polyaniline/α-Fe2O3 hybrid nanocomposites. Synth Met. 2015;204:1. https://doi.org/10.1016/j.synthmet.2015.02.032.
Li Y, Jiao MF, Zhao HJ, Yang MJ. High performance gas sensors based on in-situ fabricated ZnO/polyaniline nanocomposite: the effect of morphology on the sensing properties. Sens Actuators B Chem. 2018;264:285. https://doi.org/10.1016/j.snb.2018.02.157.
Jain S, Karmakar N, Shah A, Shimpi NG. Development of Ni doped ZnO/polyaniline nanocomposites as high response room temperature NO2 sensor. Mater Sci Eng B. 2019;247:114381. https://doi.org/10.1016/j.mseb.2019.114381.
Phuoc PH, Viet NN, Hung CM, Hoa ND, Duy NV, Hong HS, Hieu NV. Comparative study on the gas-sensing performance of ZnO/SnO2 external and ZnO-SnO2 internal heterojunctions for ppb H2S and NO2 gases detection. Sens Actuators B Chem. 2021;334:129606. https://doi.org/10.1016/j.snb.2021.129606.
Zhao SK, Shen YB, Maboudian R, Carraro C, Han C, Liu WG, Wei DZ. Facile synthesis of ZnO-SnO2 hetero-structured nanowires for high-performance NO2 sensing application. Sens Actuators B Chem. 2021;333:129613. https://doi.org/10.1016/j.snb.2021.129613.
Kang JY, Koo WT, Jang JS, Kim DH, Jeong YJ, Kim R, Ahn J, Choi SJ, Kim ID. 2D layer assembly of Pt-ZnO nanoparticles on reduced graphene oxide for flexible NO2 sensors. Sens Actuator B Chem. 2021;331:129371. https://doi.org/10.1016/j.snb.2020.129371.
Sharma B, Sharma A, Joshi M, Myung JH. Sputtered SnO2/ZnO heterostructures for improved NO2 gas sensing properties. Chemosensors. 2020;8(3):67. https://doi.org/10.3390/chemosensors8030067.
Zhang C, Sun DJ, Huan YC, Wu KD, Liao HL. Highly sensitive ZnO nanoparticles-loaded In2O3 hollow microsphere for detecting ppb-level NO2 at low working temperature. Prog Nat Sci Mater Int. 2020;30(4):469. https://doi.org/10.1016/j.pnsc.2020.06.006.
Zhao SK, Shen YB, Zhou PF, Hao FL, Xu XY, Gao SL, Wei DZ, Ao YX, Shen YS. Enhanced NO2 sensing performance of ZnO nanowires functionalized with ultra-fine In2O3 nanoparticles. Sens Actuators B Chem. 2020;308:127729. https://doi.org/10.1016/j.snb.2020.127729.
Arsha Kusumam TV, Siril VS, Madhusoodanan KN, Prashantkumar M, Ravikirand YT, Renuka NK. NO2 gas sensing performance of zinc oxide nanostructures synthesized by surfactant assisted low temperature hydrothermal technique. Sens Actuator A Phys. 2021;318:112389. https://doi.org/10.1016/j.sna.2020.112389.
Wu FY, Tseng WJ. Effect of heat treatment on surface structure and gas sensing of electrospun ZnO-SnO2 composite nanofibers. Int J Appl Ceram Tec. 2021;18(3):653. https://doi.org/10.1111/ijac.13714.
Ramgir N, Bhusari R, Rawat NS, Patil SJ, Debnath AK, Gadkari SC, Muthe KP. TiO2/ZnO heterostructure nanowire based NO2 sensor. Mater Sci Semicond Process. 2020;106:104770. https://doi.org/10.1016/j.mssp.2019.104770.
Perfecto TM, Zito CA, Volanti DP. Effect of NiS nanosheets on the butanone sensing performance of ZnO hollow spheres under humidity conditions. Sens Actuators B Chem. 2021;334:129684. https://doi.org/10.1016/j.snb.2021.129684.
Franke ME, Koplin TJ, Simon U. Metal and metal oxide nanoparticles in chemiresistors: does the nanoscale matter? Small. 2006;2(1):36. https://doi.org/10.1002/smll.200690010.
Li J, Yang M, Cheng XL, Zhang XF, Guo CY, Xu YM, Gao S, Major Z, Zhao H, Huo LH. Fast detection of NO2 by porous SnO2 nanotoast sensor at low temperature. J Hazard Mater. 2021;41:126414. https://doi.org/10.1016/j.jhazmat.2021.126414.
Liang X, Zhang J, Du LY, Zhang MZ. Effect of resonant tunneling modulation on ZnO/In2O3 heterojunction nanocomposite in efficient detection of NO2 gas at room temperature. Sensor Actuator B Chem. 2021;329:29230. https://doi.org/10.1016/j.snb.2020.129230.
Yang Y, Zhang DZ, Wang DY, Xu ZY, Zhang JH. A high-stability weighing paper/polytetrafluoroethylene-based triboelectric nanogenerator for self-powered In2O3 nanocubes/SnS2 nanoflower NO2 gas sensors. J Mater Chem A. 2021;9:14495. https://doi.org/10.1039/d1ta03739k.
Yang ZM, Zhang DZ, Chen HN. MOF-derived indium oxide hollow microtubes/MoS2 nanoparticles for NO2 gas sensing. Sens Actuators B Chem. 2019;300:127037. https://doi.org/10.1016/j.snb.2019.127037.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21771060 and 61271126), the International Science & Technology Cooperation Program of China (No. 2016YFE0115100), the Program for Science and Technology Project of Heilongjiang province (No. JQ2021B002), the Reform and Development Fund Project of Local University supported by the Central Government, Heilongjiang Touyan Innovation Team Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, QY., Yang, M., Dong, X. et al. ZnO/PANI nanoflake arrays sensor for ultra-low concentration and rapid detection of NO2 at room temperature. Rare Met. 42, 536–544 (2023). https://doi.org/10.1007/s12598-022-02149-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02149-0